Overview
This article emphasizes the mastery of electrode storage solutions, underscoring the critical importance of maintaining proper hydration and functionality of sensors, particularly pH and ORP electrodes, to ensure accurate measurements. It details various effective storage methods, including potassium chloride solutions, and outlines best practices for maintenance. Neglecting these essential practices can lead to inaccurate readings, resulting in costly consequences within laboratory settings. Therefore, it is imperative for laboratory professionals to prioritize these guidelines to uphold the integrity of their measurements.
Introduction
The accuracy and longevity of pH and ORP sensors are fundamentally dependent on the often-overlooked practice of proper electrode storage.
Specialized solutions are critical for preserving the ionic balance necessary for reliable measurements. However, many users remain unaware of the various methods available for this purpose.
What are the consequences of inadequate care for these sensors?
This article explores the essential types of electrode storage solutions, outlines best practices for maintenance, and offers troubleshooting strategies to ensure optimal performance and accurate readings in laboratory settings.
Explore the Basics of Electrode Storage Solutions
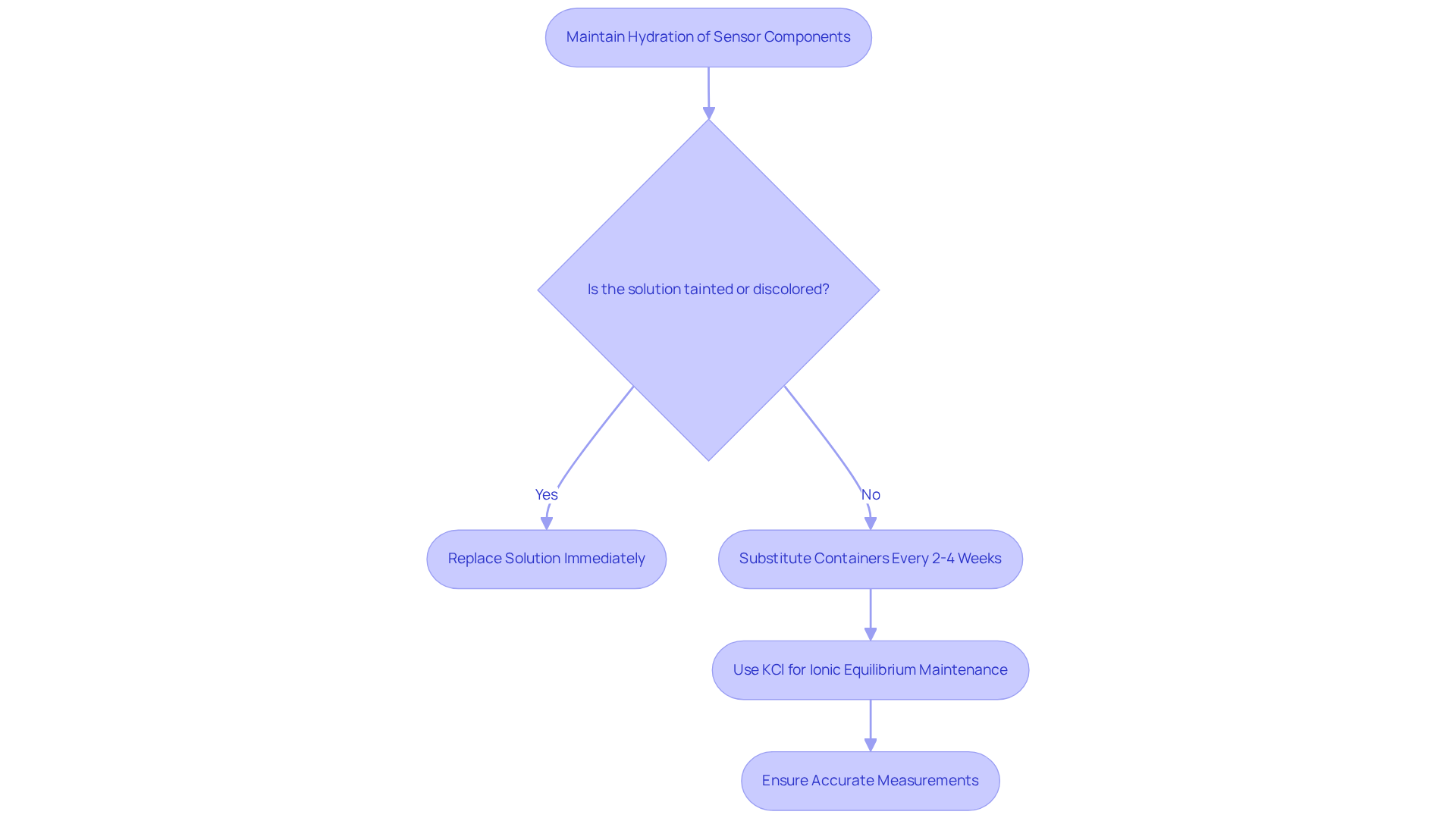
Specialized liquids are crucial for maintaining the hydration and functionality of sensor components during periods of inactivity. These methods are essential for preserving the ionic equilibrium within the conductor, preventing it from drying out, and ensuring precise measurements when reintroduced to a sample. Proper preservation is vital for extending the lifespan of sensors, particularly pH and ORP varieties, which are highly sensitive to environmental factors. Common retention methods, such as potassium chloride (KCl), play a significant role in maintaining the internal reference potential of the sensor, thus ensuring reliable readings during operation.
For example, the HI70300L pH electrode storage solution, priced at $24.99, is specifically designed to maintain hydration and prevent mineral deposits, boasting a shelf life of five years when unopened. To ensure optimal performance, it is advised to frequently substitute containers every two to four weeks, and prompt replacement is necessary if the mixture becomes tainted or discolored. Experts emphasize that maintaining the hydration of conductors is critical for achieving accurate measurements across various applications, including pharmaceuticals and environmental monitoring.
Laura French highlights the potential consequences of negligence, stating, "Inaccurate pH readings can have costly consequences such as the loss of bacterial cultures, failure of chemical reactions, and corrosion of equipment or infrastructure." Practical applications in laboratories underscore the necessity of employing suitable preservation methods to prevent degradation and ensure optimal sensor performance. Ultimately, these practices improve the reliability of analytical outcomes, reinforcing the importance of in laboratory settings.
Identify Different Types of Electrode Storage Solutions
Electrode preservation methods are essential for maintaining the integrity and functionality of pH sensors. Various types cater to specific needs and applications, including:
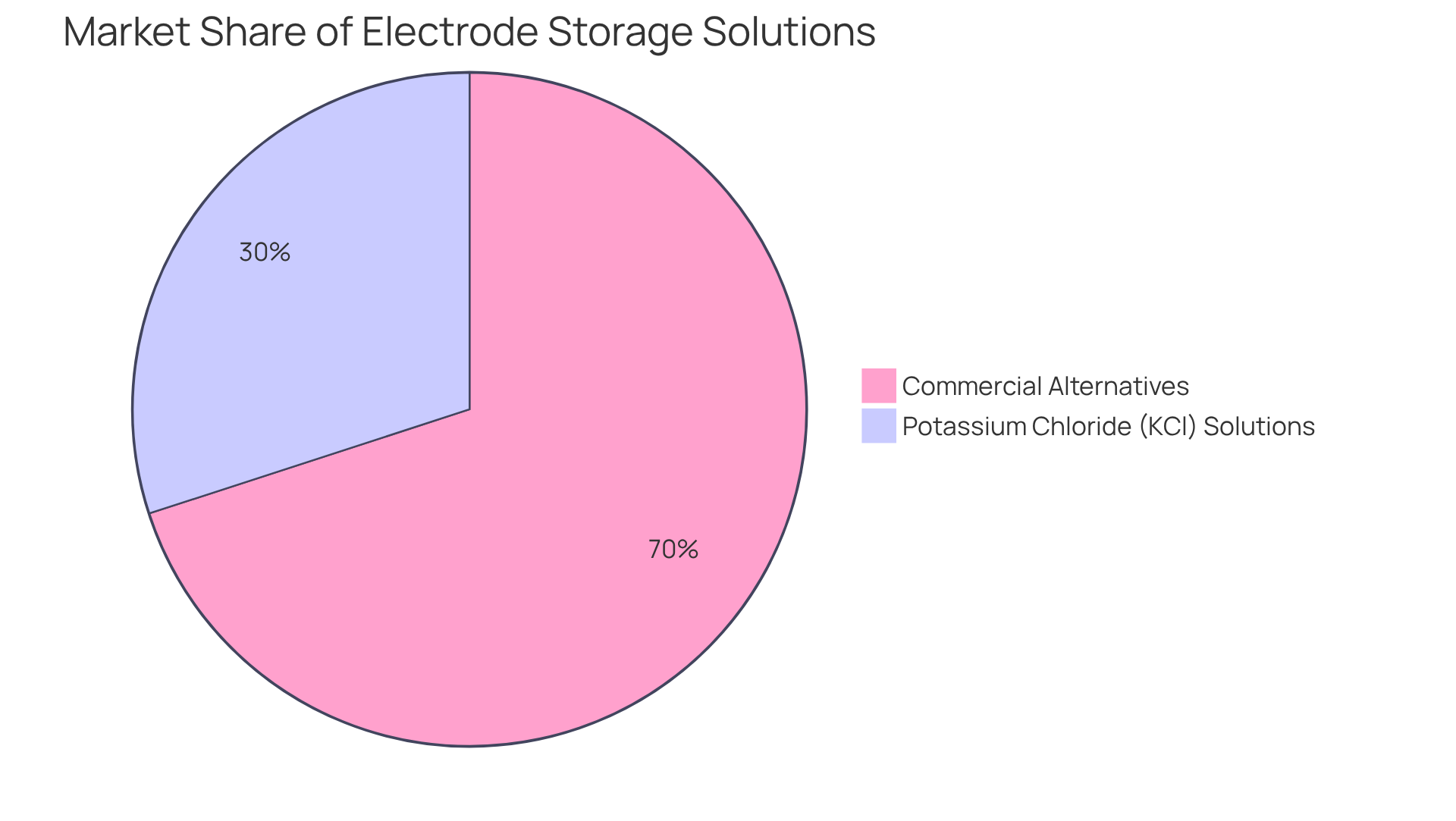
Potassium chloride (KCl) solutions are widely utilized for pH electrodes, crucial for sustaining the ionic balance necessary for accurate readings. Their efficacy is underscored by their substantial market share, which remains considerable compared to commercial alternatives, representing approximately 30% of the energy solution market.
, such as pH 4 or pH 7 buffers, serve dual purposes: they function both as calibration standards and preservation mediums. These buffers play a vital role in maintaining a stable pH environment, which is essential for specific types of sensors.
Numerous producers offer an electrode storage solution among their specialized commercial storage options designed to enhance longevity and precision. These methods often involve a mixture of substances that prevent contamination and degradation, ensuring optimal performance over time. Research has shown that devices stored in commercial mixtures exhibit a 20% extended lifespan compared to those kept in homemade mixtures.
For budget-conscious users, homemade remedies can be created using KCl and distilled water. While these methods can effectively maintain moisture in the conductors, they may lack the specialized additives found in commercial products. Consequently, homemade mixtures may be less effective in inhibiting microbial growth, which can disrupt sensor performance.
Understanding these preservation methods enables users to select the most appropriate electrode storage solution for their specific sensors, which ensures precise and reliable measurements in laboratory applications. Proper care and maintenance of pH and ORP sensors are crucial for achieving accurate measurements, necessitating the use of preservation methods to uphold their integrity and performance.
Implement Best Practices for Storing and Maintaining Electrodes
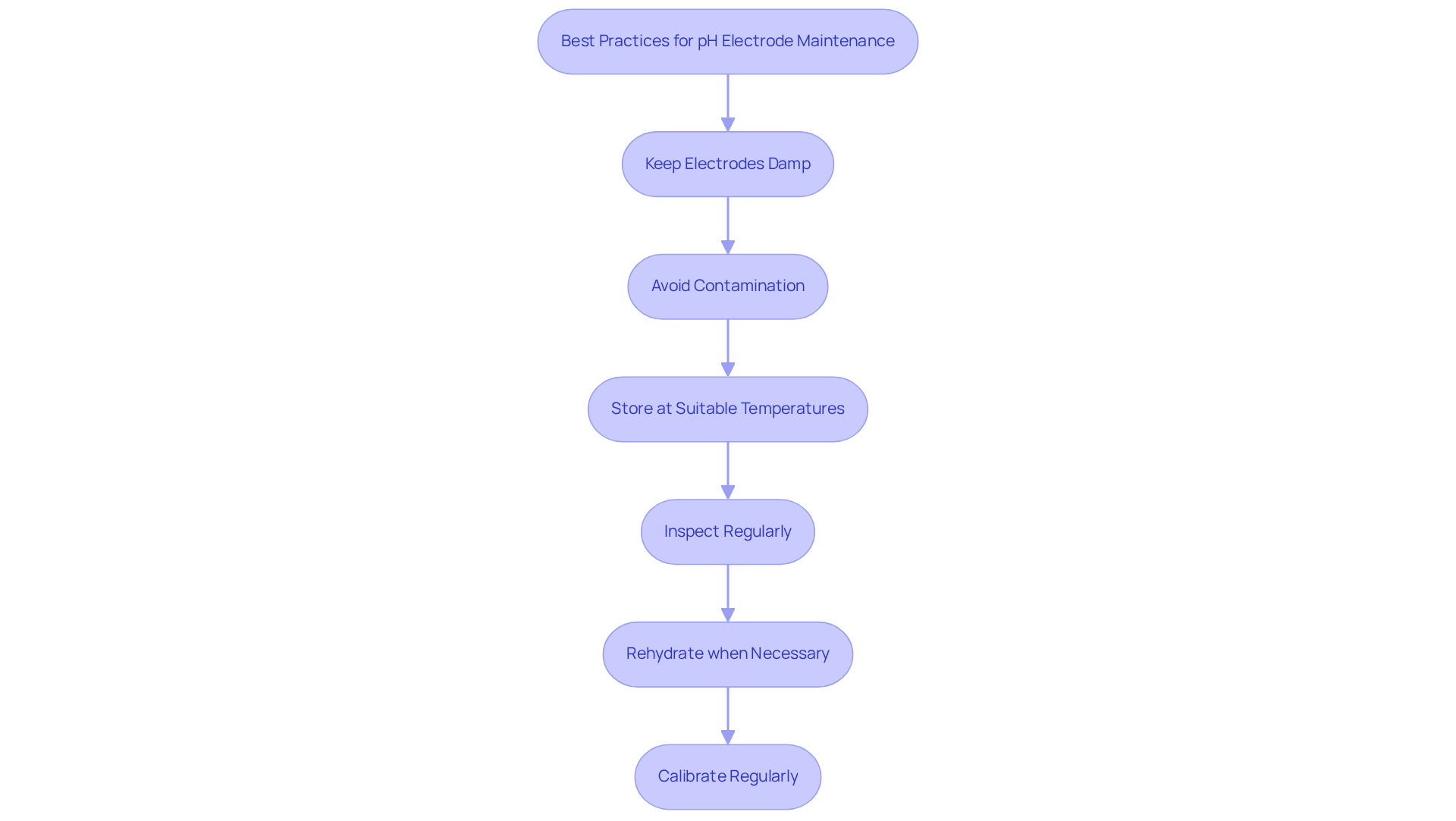
To ensure the longevity and accuracy of pH electrodes, it is imperative to adhere to and maintenance.
- Keep Electrodes Damp: Always store sensors in an appropriate preserving liquid, such as a 3M KCl mixture, to prevent drying out. A dry electrode can lead to erratic readings and inaccuracies, compromising the integrity of your measurements.
- Avoid Contamination: Ensure that the storage method remains clean and free from impurities. Regularly replacing the electrode storage solution is crucial for maintaining its effectiveness and preventing contamination that could adversely affect measurement accuracy.
- Store at Suitable Temperatures: Components should be maintained in a cool, dry environment, away from direct sunlight and extreme temperatures, which can undermine the integrity of the materials.
- Inspect Regularly: Periodically check components for signs of wear or damage. Cleaning the probe tip with appropriate solutions, such as diluted detergent for general samples or specialized cleaning agents for specific residues, is essential for maintaining optimal performance.
- Rehydrate when necessary: If a sensor has been kept dry, it should be rehydrated in the electrode storage solution for a minimum of 24 hours prior to use. This practice is crucial for restoring the component's functionality and ensuring precise measurements.
Additionally, [calibrating pH sensors](https://jmscience.com) once a month or before use, especially if they have not been used for over a month, is strongly advised. For industries requiring a high degree of accuracy, daily or weekly calibration may be more suitable. By adhering to these guidelines, laboratories can significantly enhance the functionality and reliability of their pH sensors, ultimately leading to more precise and consistent analytical outcomes. This aligns with findings from a case study highlighting the importance of regular calibration and maintenance, which emphasizes that meticulous attention to detail in maintaining pH meters results in reliable and accurate laboratory results.
Troubleshoot Common Issues with Electrode Storage
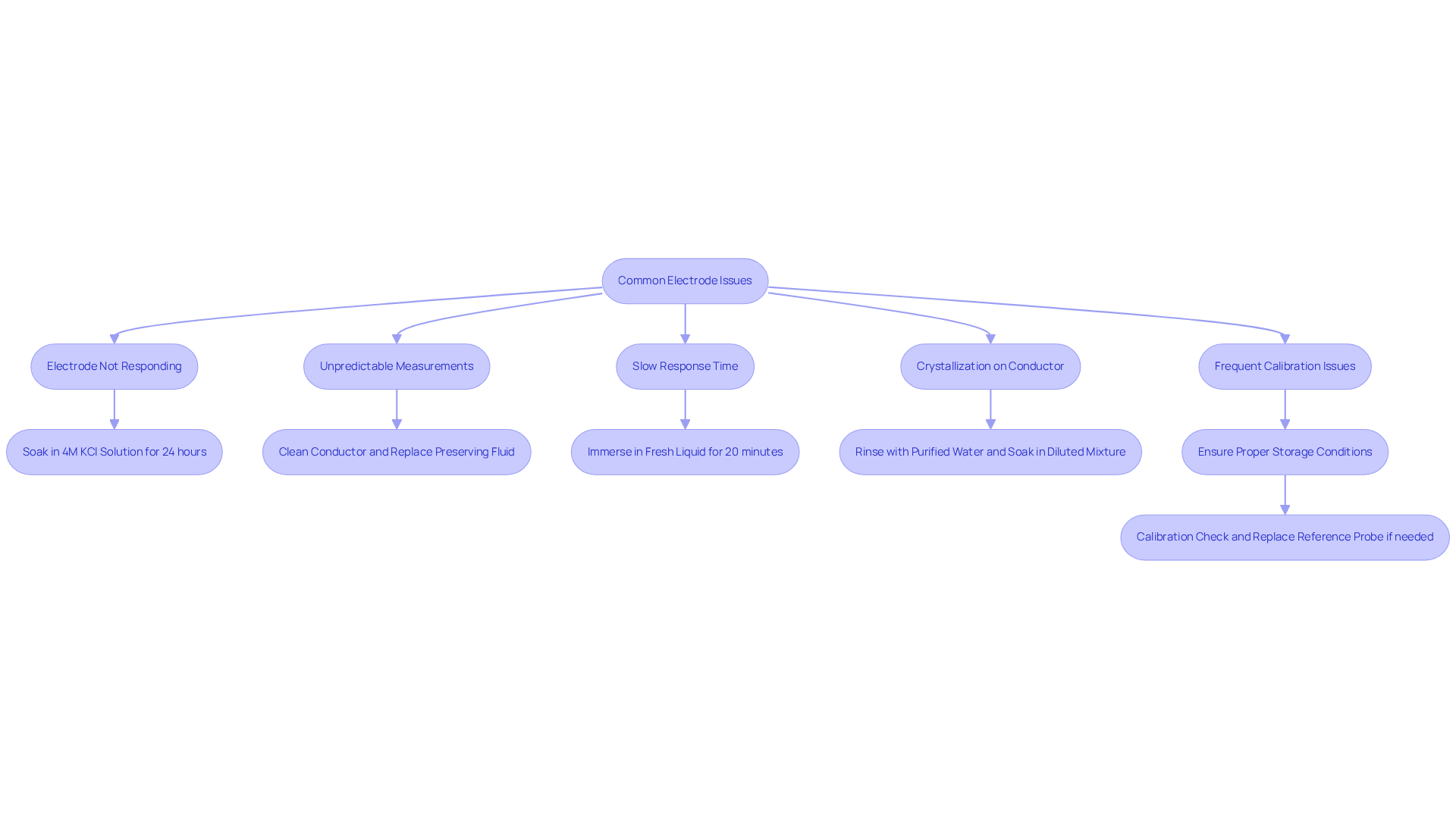
Frequent issues with the retention of these components can lead to inaccurate measurements and a reduced lifespan. To address these challenges, consider the following troubleshooting tips:
- Electrode Not Responding: If the electrode is unresponsive, it may be dry. Soak it in 4M Potassium Chloride Solution for at least 24 hours prior to use to restore its functionality.
- Unpredictable Measurements: Unpredictable measurements may occur if the sensor is contaminated or if the has been depleted. Thoroughly cleanse the conductor and replace the preserving fluid to ensure accurate readings.
- Slow Response Time: A prolonged stabilization period may indicate that the sensor requires rehydration. Immerse it in a fresh liquid medium for a minimum of 20 minutes to enhance its performance.
- Crystallization on the Conductor: The formation of crystals on the conductor suggests that the retaining fluid may be overly concentrated. Rinse the conductor with purified water and soak it in a diluted mixture to resolve this issue.
- Frequent Calibration Issues: Persistent calibration discrepancies may signal improper storage conditions. Ensure that the component is kept in the appropriate solution, and conduct regular checks. Calibration should not be necessary more than once a month in typical applications; however, weekly assessments can help identify issues early. If the offset exceeds ±30 mV, consider replacing the reference probe.
By implementing these troubleshooting practices, laboratory managers can significantly enhance the reliability and longevity of their pH electrodes, thereby ensuring accurate and consistent measurements in their analyses.
Conclusion
Mastering the intricacies of electrode storage solutions is essential for ensuring the accuracy and longevity of pH and ORP sensors. Understanding the various types of storage methods and their specific applications empowers users to make informed choices that directly impact the reliability of their measurements. The significance of proper electrode maintenance cannot be overstated; it lays the foundation for precise analytical outcomes in laboratory environments.
Key insights from the article underscore the necessity of utilizing appropriate preservation methods, such as potassium chloride solutions and pH buffers, to maintain the ionic balance and hydration of electrodes. Best practices for storage include:
- Regular inspection and rehydration
- Troubleshooting common issues
These are crucial steps in safeguarding the performance of these sensitive instruments. By adhering to these guidelines, laboratories can significantly enhance the functionality of their sensors, thereby achieving more consistent and accurate results.
Ultimately, effective management of electrode storage solutions transcends mere convenience; it is a critical factor in the success of scientific analysis across various fields. Embracing these practices will not only extend the lifespan of sensors but also bolster the integrity of the data collected, reinforcing the value of high-quality scientific instruments in delivering reliable analytical results.




