Overview
Mastering reversed phase HPLC is essential for enhancing laboratory performance, as it requires a thorough understanding of its principles, optimization of separation techniques, and strict compliance with regulatory standards. This article underscores critical advancements in:
- Method development
- Column selection
- Troubleshooting challenges
Each of these elements plays a pivotal role in achieving reliable results and maintaining high quality in pharmaceutical and biochemical analyses. By focusing on these advancements, laboratories can significantly improve their analytical capabilities and ensure the integrity of their findings.
Introduction
The realm of reversed phase high-performance liquid chromatography (RP-HPLC) stands at the forefront of analytical chemistry, offering unparalleled precision in separating and quantifying complex compounds. This sophisticated technique employs a non-polar stationary phase alongside a polar mobile phase, enabling effective differentiation between various analytes based on their hydrophobicity. As the demand for efficient and reliable analytical methods continues to rise, RP-HPLC evolves, integrating innovative materials and methodologies that enhance its performance across a wide array of applications, including pharmaceuticals and biochemistry. By exploring the principles, advancements, and practical applications of RP-HPLC, laboratory professionals can unlock the potential of this essential tool, ensuring high-quality results in their analytical endeavors.
Understanding Reversed Phase HPLC: Principles and Mechanisms
Reversed phase HPLC, a widely utilized technique, is characterized by a non-polar stationary material and a polar mobile material. This configuration facilitates the separation of compounds based on their hydrophobic characteristics; more hydrophobic compounds exhibit stronger interactions with the stationary medium, resulting in longer retention times. The fundamental principle of RP-HPLC is rooted in the partitioning of analytes between the stationary and mobile phases, a process that is significantly influenced by the polarity of the compounds being analyzed.
Recent advancements in RP-HPLC techniques have led to notable improvements in development efficiency. For example, computer-assisted method development has the potential to reduce the time required for method optimization to a mere 2–3 working days, thereby streamlining laboratory workflows. Additionally, innovative packing materials, such as the novel SiO2/SiO2 core-shell monodisperse silica spheres, have been developed through a layer-by-layer self-assembly technique. These materials exhibit enhanced carbon content and superior separation capabilities for aromatic compounds, representing a significant leap in HPLC technology.
A thorough understanding of hydrophobicity is essential for optimizing separation conditions in reversed phase HPLC. The mechanisms of separation are primarily dictated by the interactions between the analytes and the stationary phase, which can be finely adjusted by modifying the mobile phase composition. This is particularly crucial in applications involving complex mixtures, such as the analysis of monoclonal antibodies (mAbs) and antibody-drug conjugates (ADCs).
A recent case study titled "Multi-Isocratic Strategy for Protein Analysis" investigated a novel approach to protein separation that combines a multi-isocratic strategy with gradient elution. This innovative strategy allows for precise control over retention times and selectivity, resulting in uniform peak distribution and significantly higher resolution compared to traditional gradient methods. The application of this method enabled efficient separation of protein variants without the need for sample dilution and within a reduced evaluation timeframe.
In the realm of RP-HPLC, four promising substitutes for trifluoroacetic acid (TFA) have been identified, including [insert alternatives here]. However, TFA continues to be regarded as the gold standard for hydrophilic interaction liquid chromatography-mass spectrometry (HILIC-MS) evaluation. These advancements underscore the ongoing evolution of reversed phase HPLC techniques, driven by the demand for greater efficiency and precision in laboratory analyses. As industry expert Michael W. Dong emphasizes, '[insert quote here],' mastering the principles of reversed phase HPLC is vital for laboratory professionals seeking to enhance their analytical skills and achieve reliable results.
Reversed Phase vs. Normal Phase HPLC: Key Differences and Applications
Reversed phase HPLC utilizes a non-polar stationary medium in conjunction with a polar mobile medium, rendering it particularly effective for isolating polar substances. Conversely, normal mode high-performance liquid chromatography employs a polar stationary medium and a non-polar mobile medium, excelling in the separation of non-polar compounds. This method is most suitable for analytes that do not dissolve readily in water-based solvents and for compounds sensitive to aqueous environments.
The selection between these methodologies largely hinges on the chemical properties of the analytes involved. Reversed liquid chromatography is frequently employed in pharmaceutical applications due to its versatility in managing a broad spectrum of polar and moderately polar substances. This adaptability is crucial, especially since some approved methodologies necessitate reversed techniques, which may limit flexibility unless revalidated under official guidelines.
On the other hand, normal mode high-performance liquid chromatography is often the preferred choice for non-polar analytes, particularly when dealing with compounds that do not dissolve effectively in water-based solvents.
Recent trends indicate a growing preference for reversed phase HPLC in pharmaceutical analysis, driven by its efficiency and reliability. For example, a study published in the Journal of Chromatography A highlighted the separation of phenylpropionic acids utilizing both strong anion exchange and strong cation exchange stationary materials in supercritical fluid chromatography. This underscores the importance of selecting appropriate stationary materials for effective separation.
Expert insights reveal that while reversed phase HPLC is favored for its broad applicability, normal mode high-performance liquid chromatography remains relevant for specific applications, particularly where analytes are sensitive to aqueous mobile phases. User leadazide notes that refractive index (RI) detectors perform optimally with isocratic runs and are responsive to airflow and temperature changes—conditions that often align better with normal conditions. This nuanced understanding of the two techniques is vital for laboratory professionals seeking to optimize their separation strategies across various analytical contexts.
Choosing the Right Columns for Reversed Phase HPLC: A Comprehensive Guide
Selecting the appropriate columns for reversed phase HPLC is crucial for achieving optimal separation and analysis of target compounds. Key considerations include stationary material chemistry, particle size, and pore size. The most commonly employed stationary materials in reversed phase HPLC are C18, C8, and phenyl columns, each providing unique selectivity and retention characteristics.
C18 columns, for instance, are recognized for their versatility and are applicable across a wide range of uses, whereas C8 columns facilitate quicker separations, particularly for less hydrophobic compounds.
The significance of particle size on column efficiency is paramount. Smaller particles generally enhance resolution and improve peak shape; however, they also require higher operating pressures, which can impact system longevity. Recent advancements in stationary chemistry, including the development of innovative materials, have further optimized performance, allowing for improved separation and analysis of complex mixtures.
Real-world applications highlight the critical nature of column selection. A notable study on the quantification of organophosphate pesticides utilized various detectors in high-performance liquid chromatography, such as UV detectors, photodiode array detectors, and mass spectrometers. This research illustrated how the choice of stationary phase directly affected the sensitivity and accuracy of the results, underscoring the necessity of aligning column characteristics with specific evaluation needs.
Moreover, statistics indicate that over 50 additives in lubricants have been successfully isolated and measured using diverse detection techniques, showcasing the efficacy of high-performance liquid chromatography in intricate analyses. Optimal strategies for selecting chromatography columns in 2025 emphasize a comprehensive understanding of the analyte's properties and the intended separation goals. Expert recommendations suggest that laboratory professionals should evaluate the chemical characteristics of the analytes, the required detection methods, and the available instrumentation.
As one expert stated, "The choice of chromatography conditions and analytical techniques is adaptable, influenced by various factors, such as the properties of polysaccharides, the detection requirements, the availability of equipment or reagents, and the cost." By carefully assessing these factors, one can significantly enhance the efficiency and effectiveness of reversed phase HPLC methods.
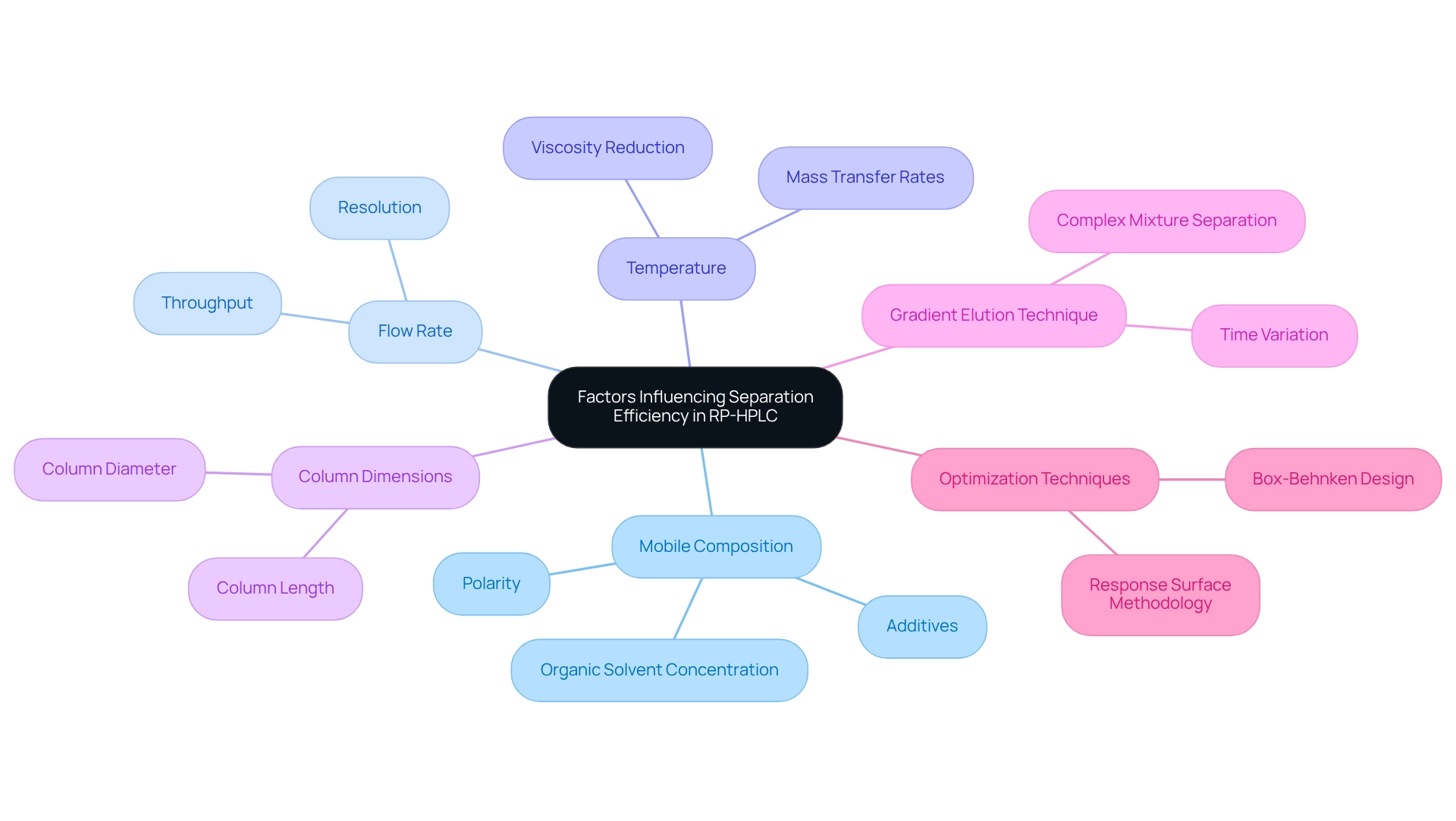
Factors Influencing Separation Efficiency in Reversed Phase HPLC
Separation efficiency in reversed state high-performance liquid chromatography (RP-HPLC) is influenced by several critical factors, including mobile composition, flow rate, temperature, and column dimensions. The polarity of the mobile medium, along with the inclusion of additives, can significantly affect retention times and peak shapes. For example, increasing the concentration of organic solvents in the moving mixture typically results in shorter retention times for hydrophobic substances, facilitating quicker evaluations without compromising resolution.
Another vital factor is the flow rate; while increased flow rates can enhance throughput, they may also diminish separation efficiency due to insufficient time for analytes to interact with the stationary medium. Conversely, lower flow rates can improve resolution but may prolong analysis times. Temperature also plays a crucial role, as elevated temperatures can lower the viscosity of the mobile phase, thereby enhancing mass transfer rates and overall separation efficiency.
Recent studies have underscored the significant impact of mobile composition on HPLC outcomes. A novel methodology for data evaluation in chromatography has emerged, employing factor analysis and hierarchical clustering to examine the effects of various parameters on compound separation. This innovative approach has minimized subjectivity in data assessment and facilitated comparisons of separations under diverse chromatographic conditions.
Further research highlights the enhancement of mobile composition in reversed phase HPLC, revealing that specific solvent mixtures can yield improved retention times and peak resolution. Notably, the application of a gradient elution technique in reversed phase HPLC—where the mobile phase composition is varied over time—has proven effective in separating complex mixtures. The generic gradient applied in preliminary screening experiments extended from 0.00 to 13.0 minutes, underscoring the importance of technique development in achieving optimal results.
Expert insights affirm that understanding these factors is crucial for laboratory professionals aiming to refine their techniques for optimal performance. As Sujatha Mahadevarao Premnath aptly states, "Quality control and laboratory safety are paramount when running chromatography for medical diagnostics." By systematically adjusting mobile mixture composition, flow rate, and temperature, laboratories can attain more reliable and reproducible results in their analytical processes.
Moreover, enhancing temperature and flow rate through a response surface approach utilizing Box-Behnken design has proven effective, providing valuable insights into optimization techniques employed in the field.
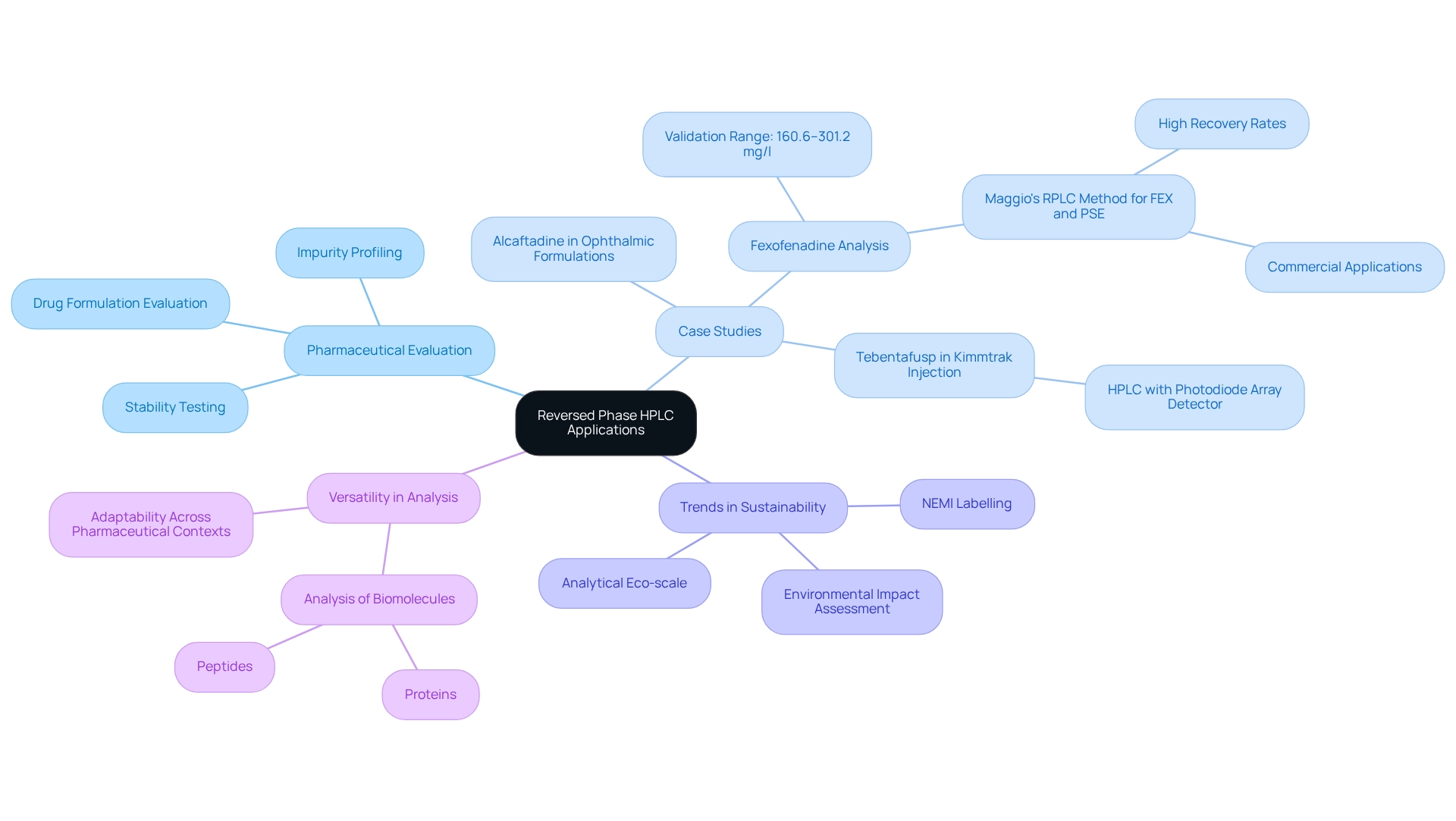
Applications of Reversed Phase HPLC in Pharmaceutical and Biochemical Analysis
Reversed phase HPLC is a fundamental technique in pharmaceutical and biochemical evaluation, recognized for its effectiveness in separating and quantifying active pharmaceutical ingredients (APIs), metabolites, and other complex compounds. This approach excels in managing intricate mixtures, rendering it especially useful for applications such as drug formulation evaluation, stability testing, and impurity profiling.
Recent statistics underscore the approach's validation for specific compounds, notably Fexofenadine (FEX), demonstrating linearity over a concentration range of 160.6 to 301.2 mg/l. This highlights its reliability in quantitative assessment. A significant case study by Maggio illustrates the successful development of a reversed phase HPLC technique for quantifying FEX and pseudoephedrine in combined tablet formulations, achieving high recovery rates and affirming its efficacy for commercial applications.
As we progress into 2025, reversed phase HPLC continues to be crucial for pharmaceutical evaluation, particularly in stability testing and formulation development. The technique is increasingly employed for the analysis of biomolecules, including proteins and peptides, facilitating their purification and characterization. Current trends reveal a growing emphasis on assessing the greenness of analytical approaches through tools such as NEMI labeling and the analytical Eco-scale, which evaluate the environmental impact of these processes.
This focus on sustainability is becoming essential to advancing analytical techniques within the pharmaceutical sector. Furthermore, reversed phase HPLC is vital for measuring active pharmaceutical ingredients, ensuring that formulations adhere to stringent quality standards. Recent discussions emphasize that the approach's versatility extends to analyzing compounds like Alcaftadine in ophthalmic formulations, showcasing its adaptability across diverse pharmaceutical contexts. Expert assessments, such as that of Prajwal S Adhav, further support this importance, highlighting the analytical technique for Tebentafusp (TEBN) in Kimmtrak Injection utilizing high-performance liquid chromatography with a photodiode array detector, underscoring the ongoing progress in liquid chromatography applications.
The versatility and adaptability of reversed phase HPLC across various analytical contexts reinforce its significance in promoting pharmaceutical research and development. It stands as an indispensable tool for laboratory professionals committed to excellence in their endeavors.
Optimizing Reversed Phase HPLC: Techniques for Enhanced Performance
To effectively enhance reversed phase HPLC methods, implementing gradient elution is essential, as it significantly improves the separation of compounds with diverse polarities. This technique involves progressively raising the concentration of organic solvents in the moving stage, facilitating a more refined separation process. Additionally, modifying the mobile mixture composition, particularly the pH, can further enhance analyte retention and improve peak shape, leading to more accurate results.
In reversed phase HPLC chromatography, detection is typically accomplished by UV absorption at 214 nm, a critical factor in procedure development.
Alongside gradient elution, adjusting temperature and flow rates plays a vital role in enhancing reversed phase HPLC performance. Recent advancements underscore the importance of controlling both the mobile and stationary phase temperatures, as illustrated in the case study on Temperature Gradient Interaction Chromatography (TGIC) of polymers. This research demonstrates how specific temperature conditions can successfully differentiate polymers, emphasizing the necessity for careful solvent choice and the potential benefits of integrating various liquid chromatography techniques.
Notably, employing non-SEC methods for the second dimension can be advantageous when polymer chain size is not the target characteristic for separation. Moreover, a contemporary computer-assisted method for optimizing separation in gradient elution has been introduced, based on polynomial estimation from preliminary experiments and a two-dimensional computer scanning technique, with resolution as the selection criterion. This highlights the ongoing development of optimization methods in the field.
Regular maintenance of the high-performance liquid chromatography system is equally crucial. This includes routine column cleaning and effective solvent management to ensure consistent performance. By systematically applying these optimization techniques, laboratory professionals can achieve more reliable and reproducible results.
Furthermore, the latest techniques for gradient elution in reversed phase HPLC, supported by statistical evidence of their efficacy, underscore the significance of these practices in enhancing analytical capabilities. As the field evolves, remaining informed about current best practices and innovative techniques will empower professionals to maximize the potential of their high-performance liquid chromatography systems. As A.W.K. Tiselius, a pioneer in chromatography, noted, the advancements in this field continue to shape our understanding and application of separation techniques.
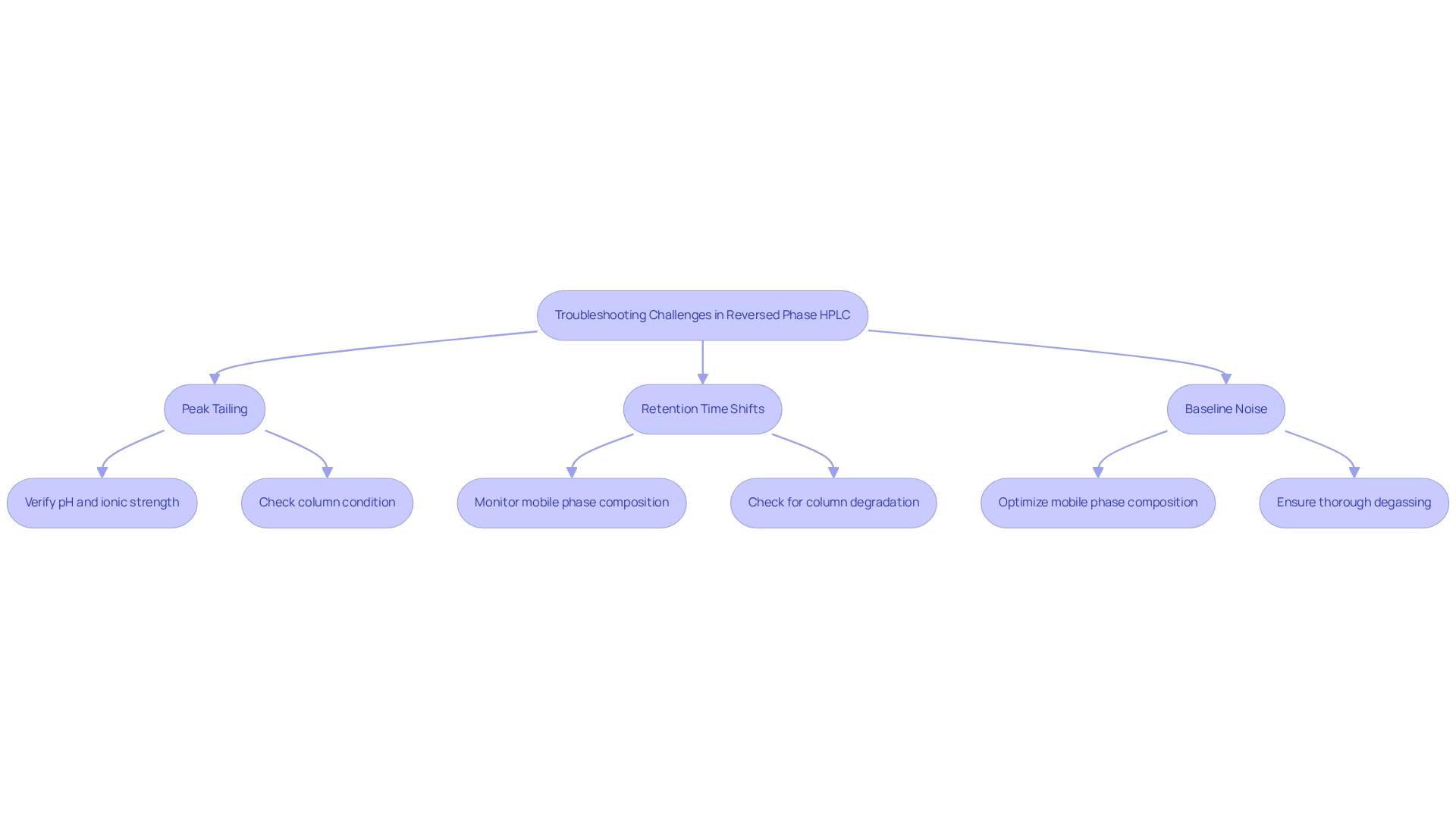
Troubleshooting Common Challenges in Reversed Phase HPLC
Reversed chromatography presents several common challenges, notably peak tailing, retention time shifts, and baseline noise. Peak tailing, a prevalent issue, can often be mitigated by ensuring that the column is properly conditioned and that the mobile medium is free of contaminants. In 2025, addressing peak tailing necessitates a systematic approach: verifying the pH and ionic strength of the mobile solution, as well as checking for any column blockages or degradation.
Retention time shifts may indicate problems with the mobile phase composition or signal column degradation; therefore, regular monitoring and maintenance are essential for effectively managing these concerns. Baseline noise, another critical challenge, can typically be minimized by optimizing the mobile phase composition and ensuring thorough degassing. Implementing these strategies not only enhances the reliability of HPLC evaluations but also improves overall data quality. Recent advancements in chromatographic techniques, such as the use of superficially porous packings (SPPs), have demonstrated potential in addressing issues like band broadening and retention time shifts, particularly in the analysis of therapeutic proteins.
The development of recombinant DNA and hybridoma technologies has led to the creation of biopharmaceutical products, which present unique challenges due to their structural heterogeneity. Progress in reversed phase HPLC paired with mass spectrometry (MS) has significantly improved the evaluation of these therapeutic proteins, facilitating enhanced characterization of monoclonal antibodies.
Statistics indicate that the segment between the elution of two peaks is frequently established at intervals of 1, 2, 8, 18, and 28 minutes, underscoring the importance of precise timing in method development and troubleshooting. By implementing contemporary strategies and leveraging real-world examples of effective troubleshooting, such as those observed in the examination of gasolines with a silica gel column, laboratory professionals can substantially enhance their high-performance liquid chromatography results, ensuring accurate and consistent outcomes in their analyses. Moreover, the emergence of Rapid Resolution Liquid Chromatography (RRLC) as a more precise and sensitive alternative to reversed phase HPLC highlights the ongoing advancements in the field, providing a broader context for the challenges encountered in reversed phase HPLC.
Ensuring Compliance and Quality Control in Reversed Phase HPLC
Ensuring compliance and quality control in reversed phase HPLC is paramount for laboratory managers, necessitating strict adherence to regulatory guidelines such as Good Laboratory Practice (GLP) and Good Manufacturing Practice (GMP). Establishing robust standard operating procedures (SOPs) for technique validation, equipment calibration, and routine maintenance is crucial. Regular audits and meticulous documentation of analytical results not only uphold compliance but also guarantee traceability, essential in pharmaceutical evaluations.
Training laboratory personnel on best practices and current regulatory requirements fosters a culture of quality and compliance, significantly impacting laboratory performance. An evaluation of 55 studies underscores that validated reversed phase HPLC techniques are vital for maintaining high standards in pharmaceutical analysis, thereby enhancing product quality and minimizing failures. For instance, Vinay Tadi remarked, "We have confirmed a reversed phase HPLC approach to precisely quantify Tofacitinib, a pharmaceutical compound," illustrating the practical application of this technique validation in real-world scenarios.
Moreover, the importance of stability-indicating techniques for evaluating Alcaftadine formulations underscores the necessity of validation in ensuring product quality.
In 2025, the latest regulatory guidelines highlight the necessity of validated methods in pharmaceutical laboratories, reinforcing the significance of compliance in safeguarding the integrity of analytical results. By prioritizing these elements, laboratories can substantially enhance their credibility and reliability in pharmaceutical testing, ultimately contributing to advancements in product quality and patient safety.
Conclusion
Reversed phase high-performance liquid chromatography (RP-HPLC) stands as a cornerstone in analytical chemistry, particularly within the realms of pharmaceuticals and biochemistry. This article has explored the underlying principles of this technique, which employs a non-polar stationary phase alongside a polar mobile phase to effectively separate compounds based on their hydrophobicity. Recent advancements, such as computer-assisted method development and innovative packing materials, have significantly enhanced both efficiency and separation capabilities.
The discussion highlighted the contrasts between reversed phase and normal phase HPLC, underscoring the critical nature of method selection tailored to analyte characteristics. Essential factors, including column selection, particle size, and mobile phase composition, were examined, illustrating their pivotal roles in optimizing separation and ensuring reliable results. The versatility of RP-HPLC is further evidenced by its applications, especially in drug formulation analysis and stability testing.
Challenges such as peak tailing and retention time shifts were addressed, with effective troubleshooting strategies presented to bolster performance. Furthermore, adherence to regulatory guidelines and quality control measures is paramount for upholding high laboratory standards.
In conclusion, mastering RP-HPLC necessitates a thorough understanding of its principles, a commitment to staying abreast of advancements, and the adherence to best practices. As the demand for precise analytical methods continues to escalate, RP-HPLC remains an indispensable tool in laboratory analysis, empowering professionals to achieve high-quality results and contribute meaningfully to pharmaceutical research and development. Its ongoing evolution emphasizes its critical role in ensuring product quality and safety within a dynamic scientific landscape.




