Overview
This article delves into the critical applications and best practices surrounding silver chloride electrodes, which play an indispensable role in electrochemical assessments due to their remarkable stability and low toxicity. By outlining their functions in both laboratory and field environments, it underscores the importance of adhering to proper storage, cleaning, calibration, and maintenance protocols. Such practices are essential for ensuring accurate and reliable performance across various applications, particularly in the realms of biomedical diagnostics and environmental monitoring. Understanding these best practices not only enhances the efficacy of these electrodes but also reinforces their value as high-quality scientific instruments in diverse settings.
Introduction
Silver chloride electrodes stand as a cornerstone of electrochemical measurements, offering a unique blend of reliability and safety across various applications. These sensors are renowned for their stable performance in both laboratory and field settings, empowering scientists and researchers to achieve precise measurements in critical assessments, ranging from environmental monitoring to biomedical diagnostics. However, despite these advantages, many users encounter challenges in maintaining optimal performance and grasping the nuances of their operation. What best practices can be implemented to ensure these vital tools deliver accurate and consistent results?
Explore the Fundamentals of Silver Chloride Electrodes
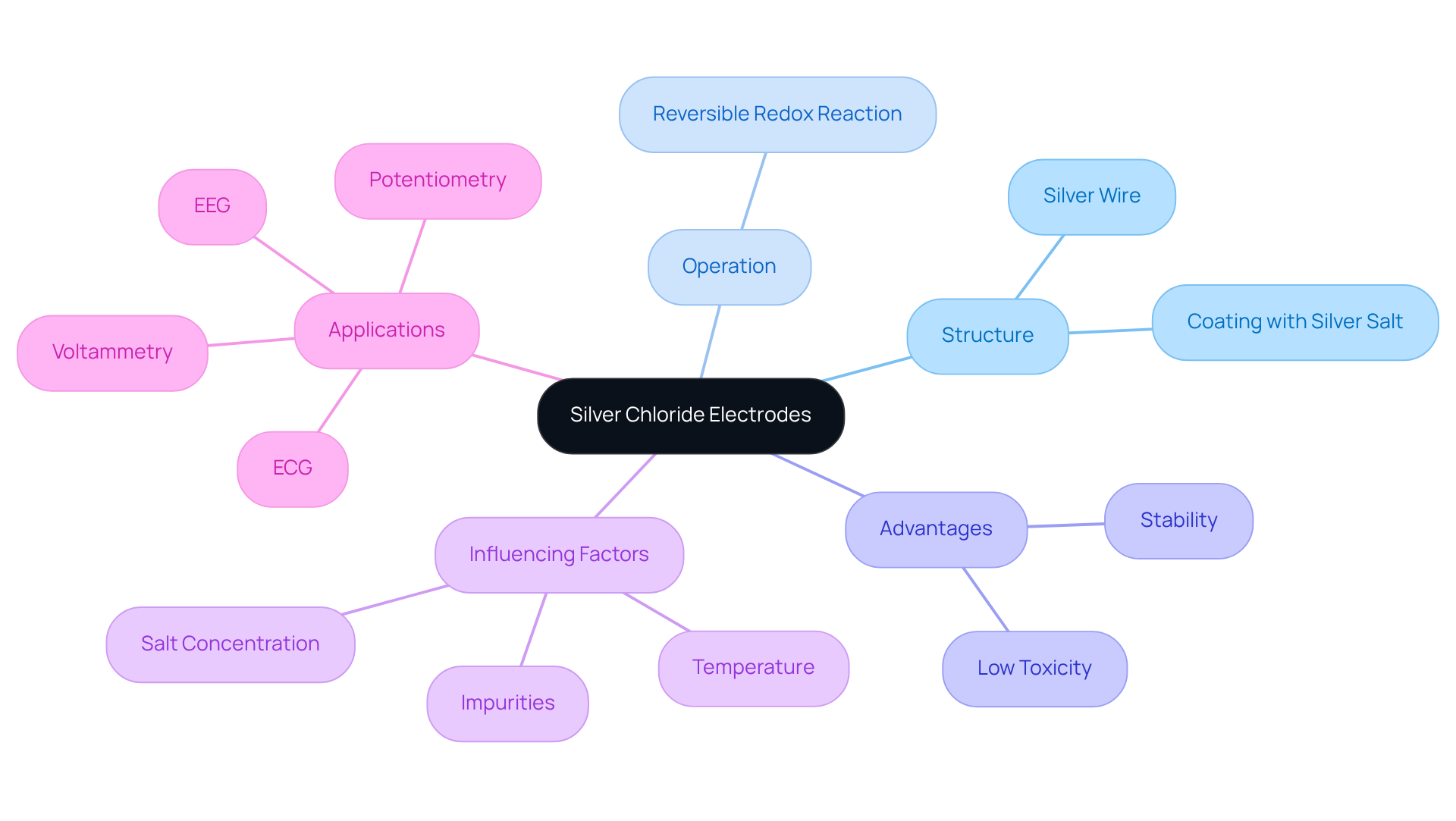
Silver bromide sensors, commonly referred to as silver chloride electrodes, represent a critical category of reference sensors frequently employed in electrochemical assessments. These sensors consist of a silver wire coated with silver salt, immersed in a salt ion solution. The operation of the device relies on the reversible redox reaction between silver ions and the silver chloride electrode, which provides a stable and reproducible potential. Such stability is paramount for achieving accurate measurements across a range of electrochemical applications, including potentiometry and voltammetry.
Significantly, sensors that incorporate a silver chloride electrode are preferred due to their low toxicity compared to calomel types, making them safer for laboratory environments. However, the performance of these sensors can be influenced by various factors, including temperature, salt concentration, and the presence of impurities. Notably, the temperature coefficient at room temperature is approximately +0.214 mV/°C, underscoring the necessity of temperature regulation to maintain measurement accuracy. Furthermore, silver chloride electrode sensors demonstrate effectiveness even at lower concentrations, such as 1 M potassium chloride, which enhances their versatility across different settings.
In laboratory contexts, the silver chloride electrode is extensively utilized due to its reliability and efficiency, establishing it as an essential instrument in electrochemical studies and diagnostics, including applications in electrocardiography (ECG) and electroencephalography (EEG). Their stability under ionizing radiation further emphasizes their dependability for specific applications, particularly in nuclear-related environments.
Examine Applications in Laboratory and Field Settings
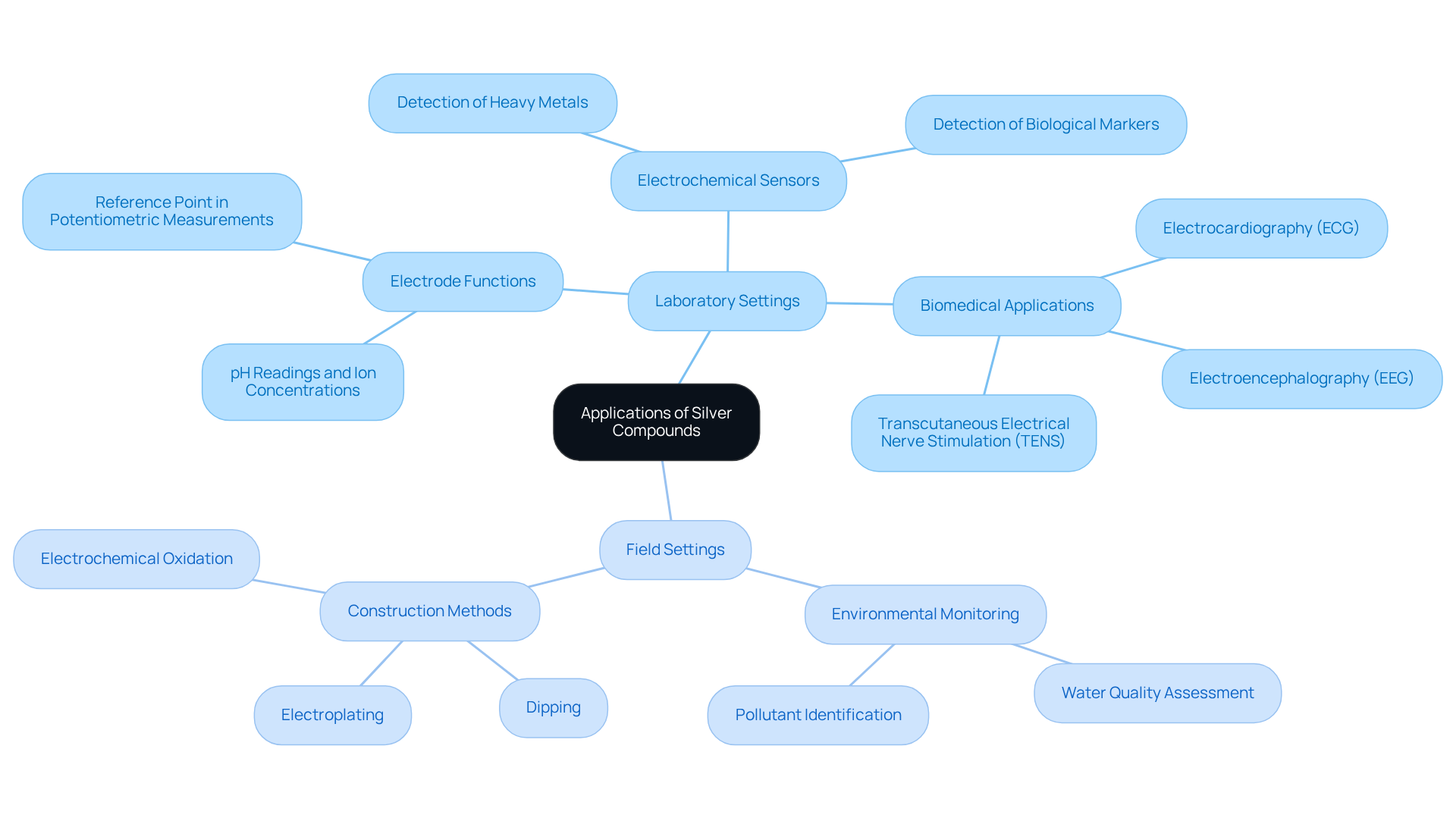
Silver compounds play a crucial role in various applications across both laboratory and field environments. In laboratory settings, the silver chloride electrode acts as a reference point in potentiometric measurements, ensuring precise pH readings and accurate determinations of ion concentrations. Their utility extends to electrochemical sensors, where they adeptly detect a wide range of analytes, including heavy metals and biological markers, which underscores their versatility in analytical chemistry. Notably, the potential of the silver chloride electrode Ag/AgCl/saturated KCl is +0.197 V, and the half-cell potential of the silver chloride electrode in silver/silver chloride cells is approximately +222 mV (SHE), highlighting their efficiency in these applications.
In field applications, silver chloride electrodes like Ag/AgCl sensors excel in environmental monitoring, effectively assessing water quality and identifying pollutants. Their inherent stability and low toxicity render them suitable for long-term deployments in challenging conditions, such as soil and water analysis. The construction of commercial reference devices typically involves methods like dipping, electroplating, or electrochemical oxidation, all of which contribute to their reliability. Additionally, a porous filter at the tip of the reference sensor establishes liquid contact between the measured solution and the electrolyte solution, thereby enhancing their functionality.
These components are indispensable in biomedical applications, particularly in electrocardiography (ECG) and other biosignal measurements, where their reliable performance is paramount. The use of the silver chloride electrode in these contexts not only facilitates precise biomonitoring but also minimizes toxicity, making it a preferred choice in medical diagnostics and patient monitoring systems. As illustrated in the case study on silver/silver salt sensors in biomonitoring, their efficient transformation of ion current at human tissue surfaces into electron current is critical for achieving accurate measurements.
Implement Best Practices for Using Silver Chloride Electrodes
To ensure optimal performance of the silver chloride electrode sensors, several best practices should be followed.
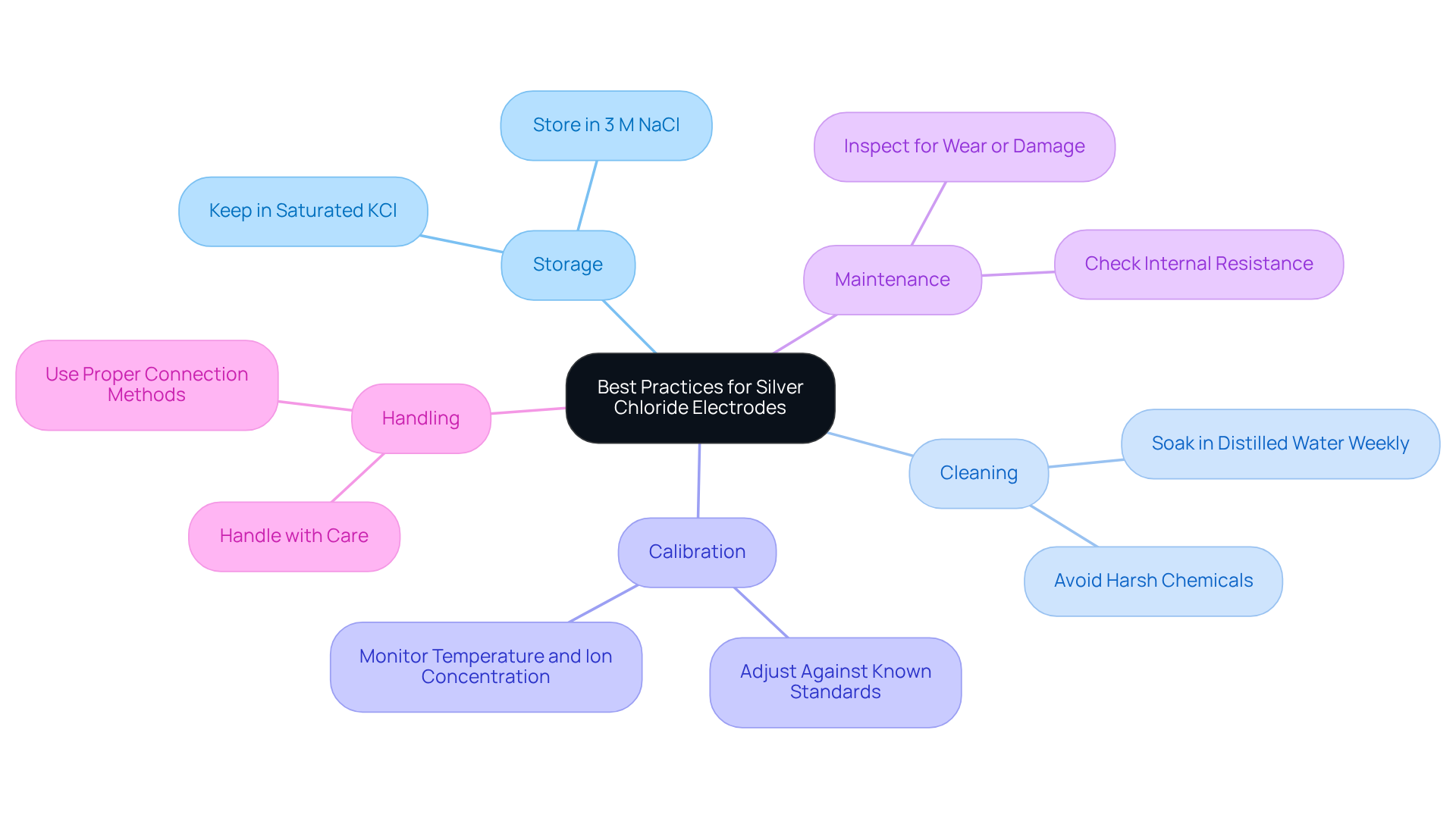
Storage: Always keep Ag/AgCl sensors in a saturated KCl solution to prevent drying out, which can lead to performance degradation. This practice is essential, as devices that dry out can experience irreversible damage. Additionally, it is advisable to store reference devices in 3 M NaCl when not in use to further enhance their longevity.
Cleaning: Regularly clean the surface of the device with distilled water to remove contaminants. It is essential to avoid harsh chemicals that could jeopardize the integrity of the component. Weekly soaking in distilled water helps maintain cleanliness and functionality. Case studies have demonstrated that keeping the conductive paste moist and preventing it from drying out is crucial for sustaining performance.
Calibration: Periodically adjust the sensors against known standards to ensure accuracy. Calibration is crucial, as the potential of the silver chloride electrode can be affected by temperature variations and the concentration of chloride ions in the solution. As mentioned, "Proper maintenance, including regular refilling of the KCl solution and cleaning of the sensor, is essential to ensure long-term stability and accuracy." Neglecting calibration can lead to significant errors in readings, emphasizing its importance in laboratory settings.
Maintenance: Examine the components for indications of wear or damage, and replace them if needed. Regular internal resistance checks should be performed, ideally keeping resistance below 10KΩ to prevent blockages that can compromise performance.
Handling: Manage the conductors with care to prevent physical harm, and always apply suitable methods when linking them to testing devices. Proper handling minimizes the risk of introducing noise or mistakes during assessments.
By adhering to these best practices, users can maximize the reliability and longevity of their silver chloride electrodes, which ensures accurate and stable electrochemical measurements.
Conclusion
Silver chloride electrodes, also known as silver bromide sensors, are indispensable tools in the field of electrochemical assessments, merging safety with efficiency for dependable measurements. Their distinctive construction and operational principles enable them to provide stable potentials, a crucial factor for accurate data across a spectrum of applications, from laboratory experiments to field monitoring of environmental conditions.
This article has delved into the diverse applications of silver chloride electrodes, underscoring their essential roles in laboratory contexts—such as potentiometric measurements and analytical chemistry—as well as in field environments, including environmental monitoring and biomedical diagnostics. The discussion on best practices highlighted the necessity of proper storage, cleaning, calibration, and maintenance to optimize the performance and longevity of these sensors, thereby ensuring their reliability in delivering precise measurements.
In conclusion, the importance of silver chloride electrodes is paramount, as they effectively bridge the gap between safety and functionality in electrochemical applications. By adhering to the recommended practices, users can significantly enhance the accuracy and stability of their measurements, ultimately fostering advancements in research and diagnostics. Embracing these best practices not only improves the performance of silver chloride electrodes but also encourages innovation in the fields where they are utilized, promoting further exploration and application of this crucial technology.




