Overview
This article delves into the principles and applications of electrochemical detectors, underscoring their operational mechanisms and the diverse types utilized across various fields. A thorough understanding of redox reactions, electrode types, and calibration methods is paramount for achieving accurate measurements and maximizing the effectiveness of applications in critical areas such as environmental monitoring and healthcare diagnostics. By exploring these foundational concepts, we establish the significance of high-quality scientific instruments and their impact on laboratory settings.
Introduction
Electrochemical detection is at the forefront of analytical chemistry, offering essential insights across diverse fields, from environmental monitoring to healthcare diagnostics. By mastering the fundamental principles and varied applications of electrochemical detectors, users can unlock the potential for precise and reliable measurements. Yet, with numerous detector types available, each possessing unique strengths and limitations, how can one effectively navigate the complexities of selection, setup, and calibration to achieve optimal performance?
Explore the Principles of Electrochemical Detection
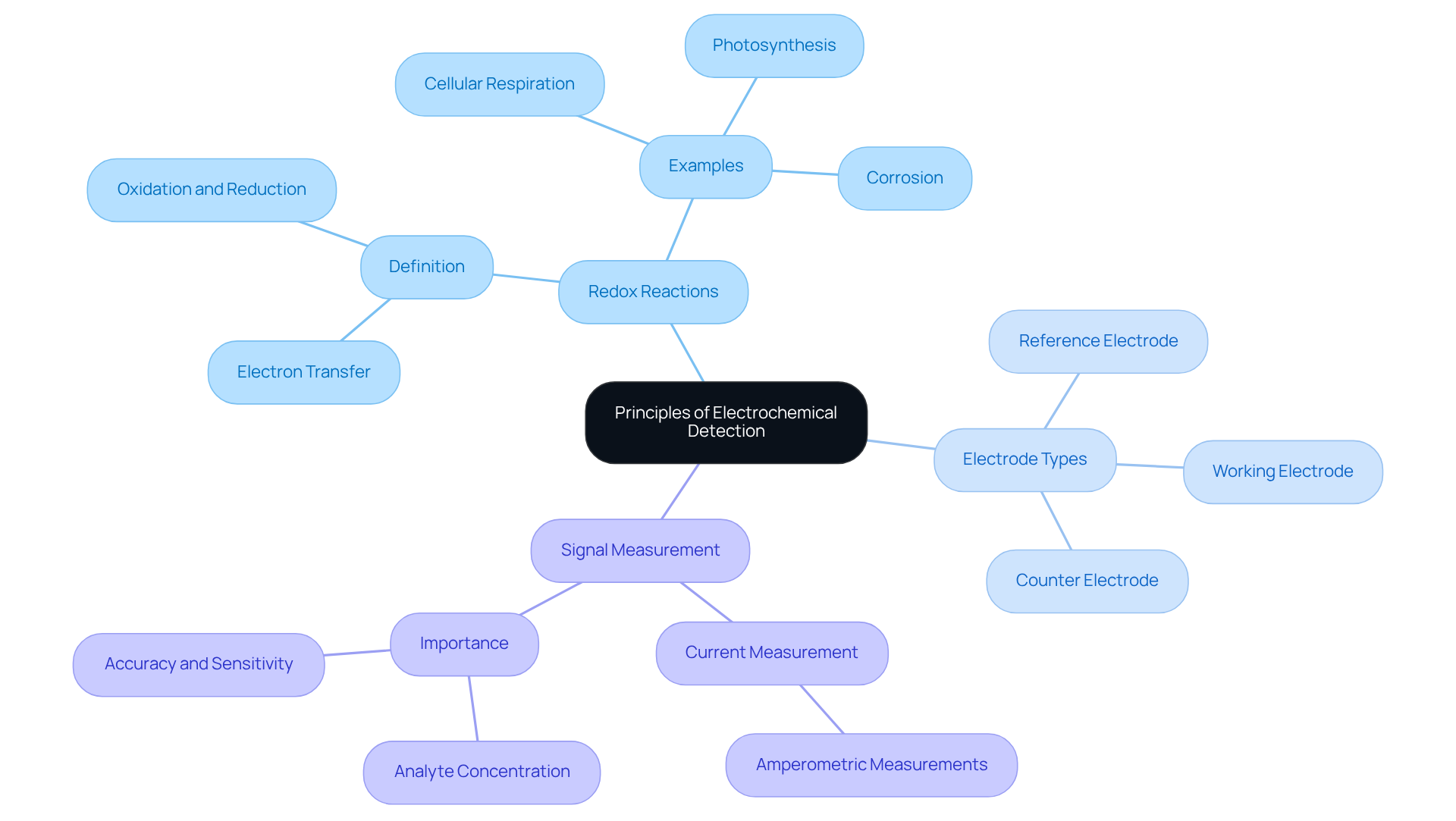
An electrochemical detector operates through the interaction between chemical species and conductive surfaces, generating an electrical signal in response to chemical reactions. This fundamental principle involves measuring changes in current or potential resulting from oxidation or reduction reactions occurring at the conductor's surface. Various factors—including the nature of the analyte, electrode material, and applied potential—can significantly influence this process. A comprehensive understanding of these principles is essential for enhancing identification techniques and ensuring accurate outcomes in analytical applications.
Key concepts include:
- Redox Reactions: Central to electrochemical detection, these reactions entail the transfer of electrons between species, which is critical for the detection process. Notable examples include cellular respiration, photosynthesis, and corrosion, underscoring the significance of redox reactions in both biological and industrial contexts.
- Electrode Types: Different electrodes, such as working, reference, and counter electrodes, each serve specific roles in the identification process, thereby enhancing the overall effectiveness of the system.
- Signal Measurement: The current generated during the reaction is quantified, yielding vital data regarding analyte concentration, which is crucial for precise analysis. Amperometric measurements, renowned for their high accuracy and sensitivity, are frequently utilized in this context.
Electrochemical sensors, including the electrochemical detector, fulfill diverse roles such as environmental monitoring to identify contaminants like heavy metals and gases such as CO, NOx, and SO2, as well as their application in healthcare for disease diagnosis and monitoring. By mastering these principles, users can effectively troubleshoot and enhance their electrochemical detection setups, leading to improved performance across various domains, including environmental monitoring and healthcare diagnostics.
Identify Different Types of Electrochemical Detectors and Their Applications
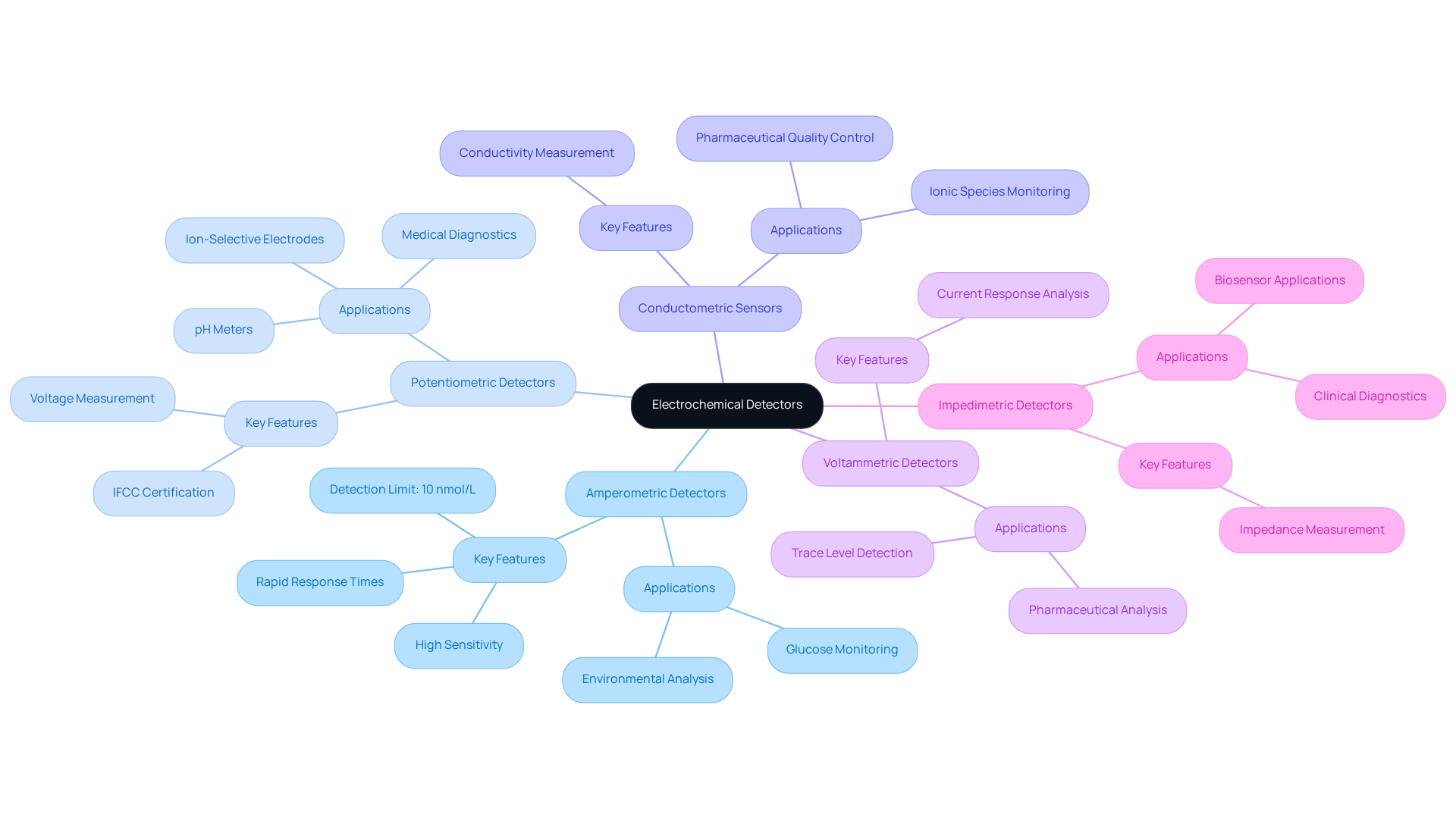
Electrochemical detectors are classified into several types, each designed for specific applications, highlighting their importance in various fields.
-
Amperometric Detectors measure the current generated by the oxidation or reduction of analytes. This makes them particularly suitable for applications such as glucose monitoring and environmental analysis. Their high sensitivity, with detection limits as low as 10 nmol/L for methimazole, combined with rapid response times, offers significant advantages in pharmaceutical settings where precise measurements are critical, particularly when utilizing an electrochemical detector.
-
Potentiometric Detectors gauge voltage changes in response to variations in ion concentration. Extensively utilized in medical diagnostics, the electrochemical detector is essential in pH meters and ion-selective electrodes, which monitor physiological parameters and ensure accurate drug formulations. The certification of these sensors by the International Federation of Clinical Chemistry (IFCC) for various ions further enhances their credibility in clinical applications.
-
Conductometric Sensors evaluate variations in conductivity within a solution, proving effective for monitoring ionic species across diverse applications, including environmental testing and pharmaceutical quality control.
-
Voltammetric Detectors analyze the current response as a function of applied voltage. Their effectiveness in detecting trace levels of substances in complex matrices is invaluable, particularly in pharmaceutical analysis, where they enable the detection of low-concentration drugs and metabolites, thereby improving the reliability of analytical results.
-
Impedimetric Detectors measure the impedance of a solution, providing valuable insights into the electrochemical properties of analytes. Their increasing prevalence in biosensor applications, especially for tracking biomolecules in clinical diagnostics, underscores their growing significance.
Each type of electrochemical detector presents distinct benefits and drawbacks, underscoring the importance of selecting the appropriate device based on specific analytical needs and the characteristics of the samples being examined. For instance, amperometric sensors are preferred for their rapid analytical speed and low detection limits, whereas potentiometric instruments excel in applications necessitating precise ion concentration measurements. Notably, the amperometric sensing market is projected to reach approximately $1.4 billion by 2033, indicating substantial growth potential in this field.
Understand the Setup and Calibration of Electrochemical Detectors
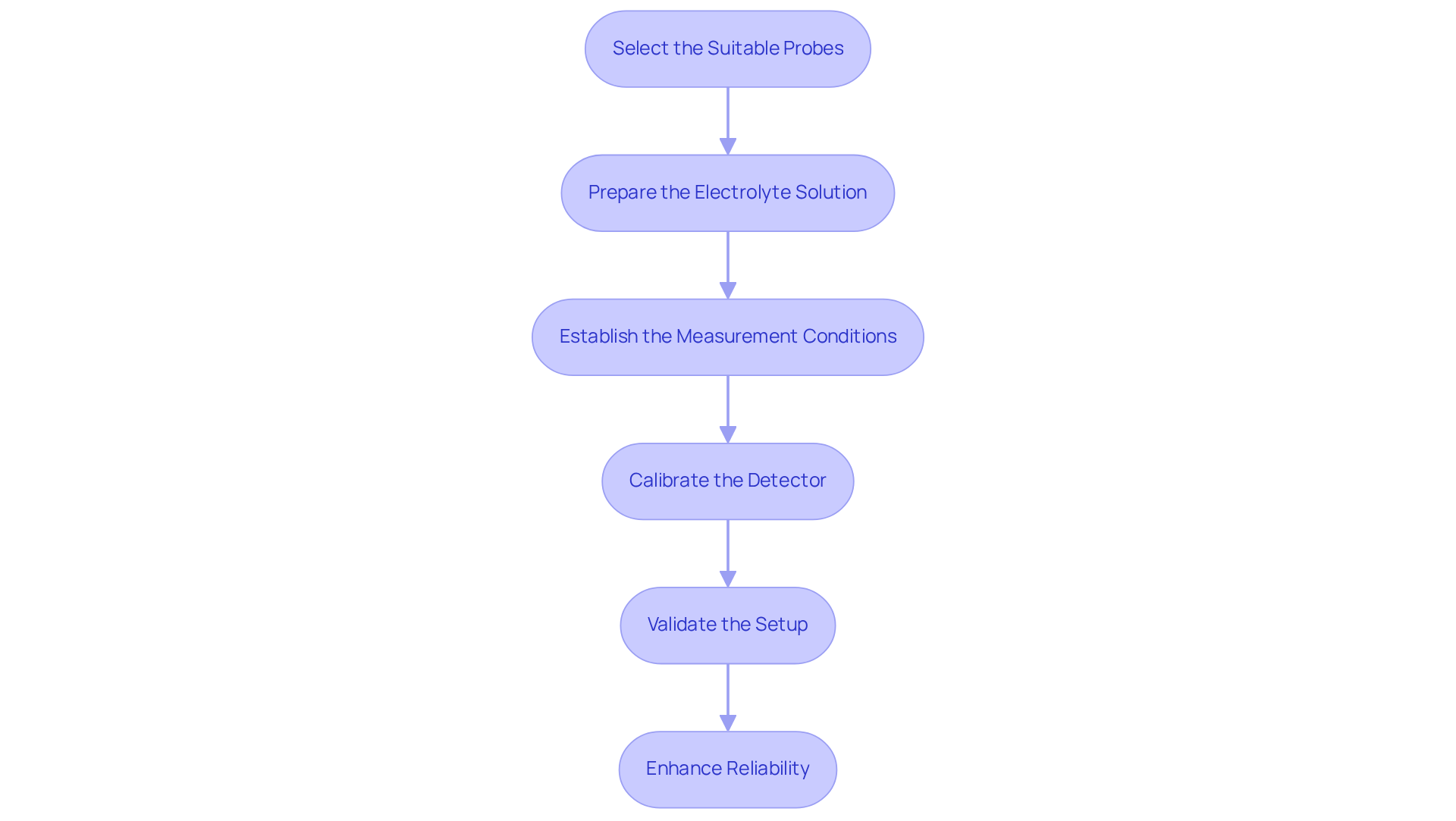
Setting up and calibrating an electrochemical detector involves several essential steps that are crucial for ensuring optimal performance.
-
Select the Suitable Probes: Begin by choosing probes that are specifically customized for the analysis and characteristics of the analyte. It is imperative to ensure that these probes are clean and well-maintained to avoid contamination. As Riken Keiki emphasizes, "The gas concentration should be proportional to the current value," underscoring the significance of proper electrode selection in achieving accurate measurements.
-
Prepare the Electrolyte Solution: Next, utilize an electrolyte that aligns with the requirements of both the sensing device and the analyte. This step is vital for obtaining precise measurements. The ideal environmental conditions for electrochemical sensors are a temperature of 20°C and relative humidity between 60% to 80%. These factors can significantly influence sensor performance, making careful preparation essential.
-
Establish the Measurement Conditions: It is also important to set the temperature, pH, and other environmental factors that may impact the detection process. These conditions must remain within the optimal range for the specific application. High temperatures can lead to the evaporation of the electrolyte, which adversely affects sensor accuracy, as noted by Riken Keiki.
-
Calibrate the Detector: Calibration is a critical step where standard solutions of known concentrations are employed. Assessing the sensor's response to these standards allows for the establishment of a dependable calibration curve, which is essential for precise quantification. Notably, calibration using a nonparametric algorithm has demonstrated RMSE values greater than 0.997 across a wide dynamic range, highlighting the effectiveness of proper calibration methods.
-
Validate the Setup: Finally, conduct initial tests using known samples to confirm that the device is functioning correctly and yielding accurate results. This validation step is critical for establishing confidence in the measurement process. Engaging trained professionals for calibration and testing is crucial for ensuring sensor reliability, particularly when using an electrochemical detector, as supported by various case studies.
Adhering to these steps will significantly enhance the reliability and accuracy of the electrochemical detector in various analytical applications.
Conclusion
Understanding the intricacies of electrochemical detectors is essential for enhancing analytical capabilities across various fields. By grasping the fundamental principles, types, and calibration processes of these detectors, users can significantly improve their analytical outcomes. The importance of electrochemical detection in applications ranging from environmental monitoring to healthcare diagnostics cannot be overstated, as it provides crucial insights into the presence and concentration of various analytes.
Key insights from this article highlight the significance of:
- Redox reactions
- The diverse types of electrochemical detectors
- The meticulous setup and calibration required for optimal performance
Each type of detector—amperometric, potentiometric, or voltammetric—offers unique advantages tailored to specific applications, emphasizing the need for careful selection based on analytical requirements. Furthermore, the calibration process is critical for ensuring accuracy and reliability in measurements, reinforcing the importance of adhering to best practices.
In light of the growing demand for precise analytical techniques, embracing the principles and applications of electrochemical detection is more relevant than ever. As advancements in technology continue to emerge, staying informed about the latest developments will empower users to leverage these tools effectively. Whether in environmental science, healthcare, or industrial applications, mastering electrochemical detection techniques is vital for achieving reliable results and driving innovation in analytical chemistry.




