Overview
The fundamental distinctions between titrant and titrand are pivotal in the titration process. The titrant, a solution of known concentration, is introduced to the titrand, which contains the analyte of unknown concentration. Recognizing these differences is essential for achieving precise quantitative analysis. The titrant plays a crucial role in measuring the concentration of the titrand through their chemical interaction. This interaction significantly influences the reliability of laboratory results, underscoring the necessity for a thorough understanding of these components in scientific practices.
Introduction
In the intricate realm of analytical chemistry, grasping the roles of titrant and titrand is paramount for achieving precise measurements and analyses. These components not only engage in the titration process but also fulfill distinct functions that critically influence experimental outcomes.
As researchers navigate the complexities of titration, a vital question emerges: how does the selection and application of these substances affect the reliability of analytical results?
By examining the differences between titrant and titrand, one uncovers essential insights that can refine laboratory practices and ensure accuracy in quantitative assessments.
Define Titrant and Titrand: Core Concepts in Titration
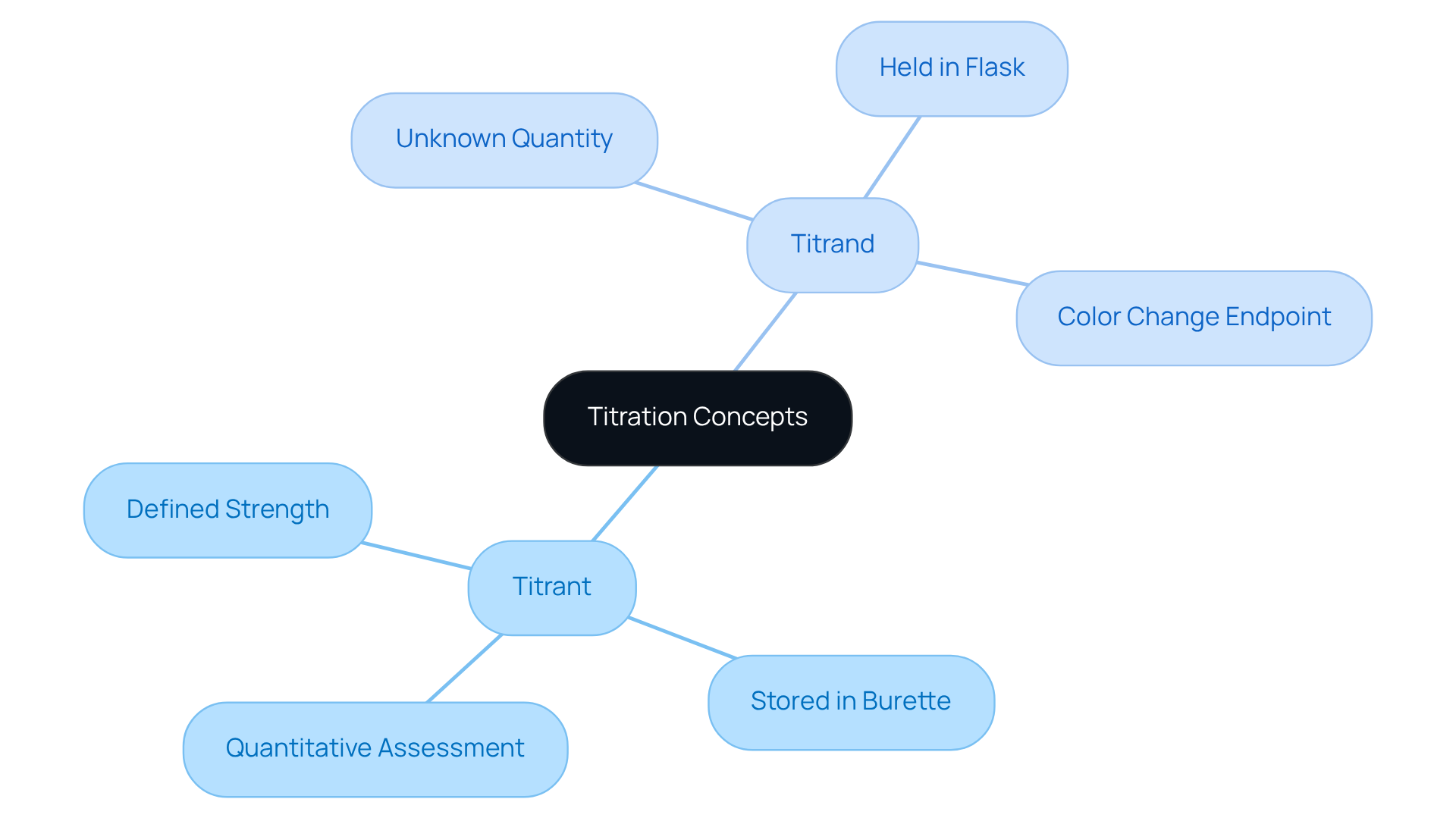
In the realm of analytical chemistry, the measurement process is pivotal. The reagent, known as the titrant, represents a solution with a defined strength, methodically added to another solution in the context of titrant vs titrand to ascertain its strength. Typically, this titrant is stored in a burette, allowing for precise dispensing throughout the titration process. In contrast, the substance, often referred to as the titrand, contains an unknown quantity, highlighting the difference in roles when discussing titrant vs titrand, and is held within a flask.
The interaction between these two solutions facilitates a quantitative assessment of the analyte's amount, emphasizing the importance of understanding the concepts of titrant vs titrand for experts in the field. This foundational knowledge is critical for effective laboratory practices and accurate analytical results.
For instance, in acid-base reactions, the reagent engages with the sample, reaching an endpoint that is visually indicated by a color change. This phenomenon not only verifies the analyte's amount but also highlights the essential nature of these interactions in achieving reliable outcomes in laboratory settings.
Examine Functions: Roles of Titrant and Titrand in Titration
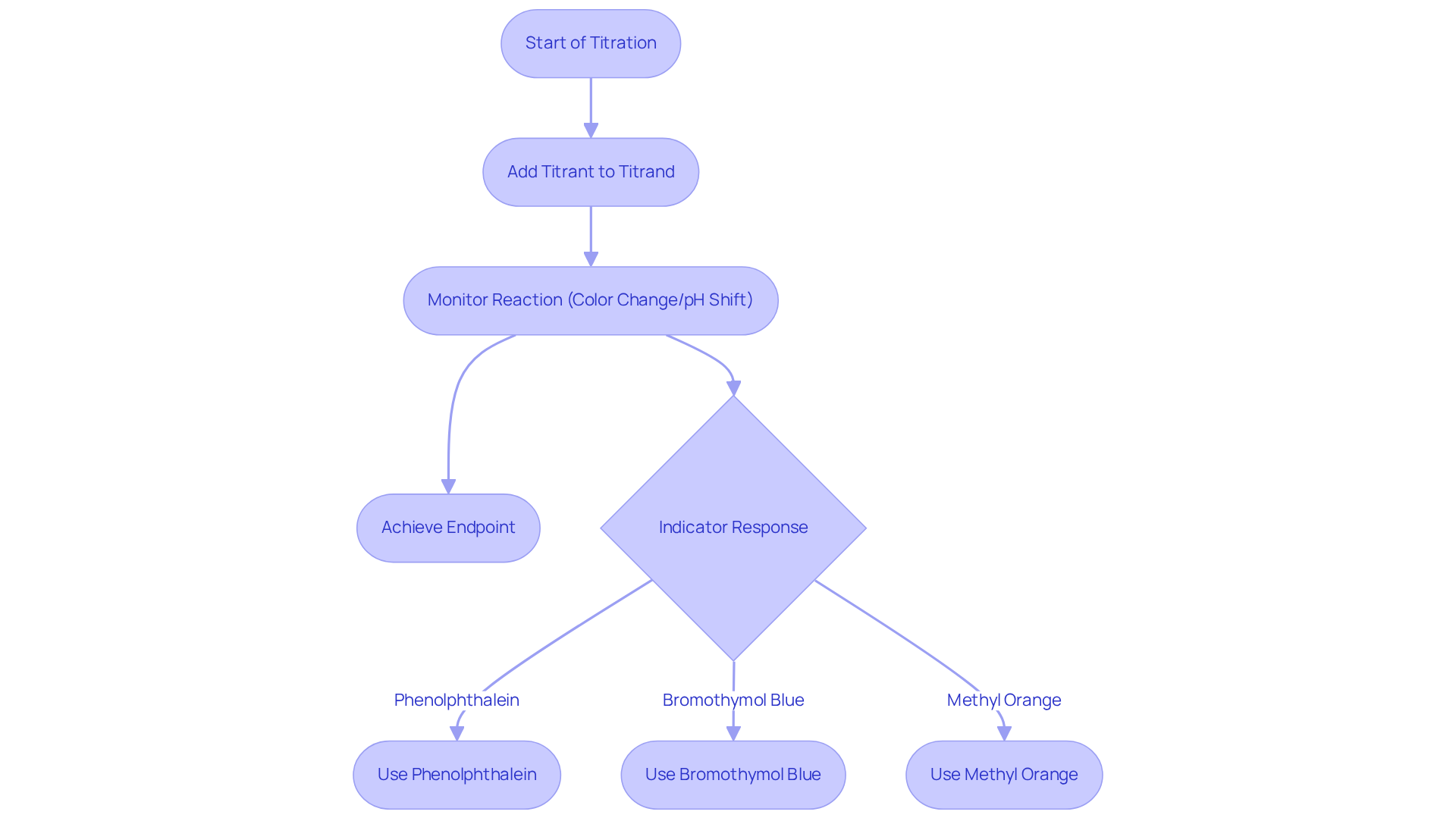
The substance acts as the reagent, illustrating the concept of titrant vs titrand as it interacts with the analyte to achieve the endpoint of the titration. With its level known, precise computations of the reagent's concentration can be made based on the volume of solution utilized. Conversely, the solution represents the substance under analysis as the titrand, while its concentration is the focal point of the experiment in the context of titrant vs titrand. Throughout this process, the reagent is incrementally added to the analyte until a reaction occurs that signifies the endpoint, often marked by a color change or a shift in pH. This interaction is vital for obtaining accurate and reliable results in quantitative analysis.
For instance, in a standard acid-base titration, the choice of indicator—such as phenolphthalein or bromothymol blue—is crucial, as it must respond appropriately to the pH fluctuations that occur as the reagent is introduced. The concentration of the reagent plays a significant role in the outcomes of the analysis; a higher concentration can expedite the approach to the endpoint, while a lower concentration may necessitate a greater volume of reagent to achieve the same result, potentially introducing variability in measurements. Understanding these dynamics is essential for implementing effective dosing practices.
Evaluate Applications: Choosing the Right Titrant and Titrand for Laboratory Needs
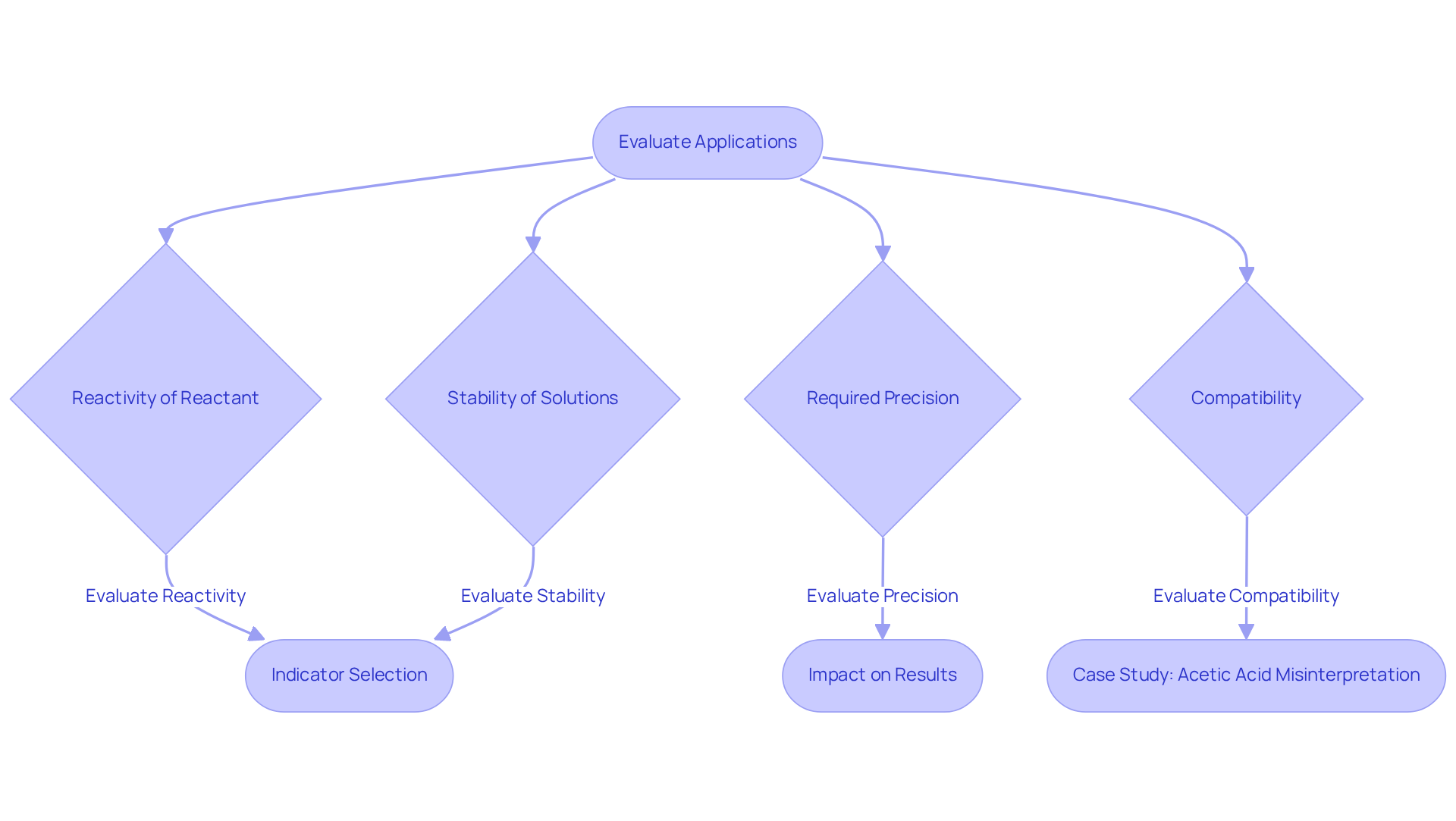
Selecting the appropriate reagent and analyte is crucial for achieving precise and reliable results in laboratory analyses. Key factors to consider encompass the reactivity of the reactant with the analyte, the stability of the solutions, and the required precision of the measurements. For example, in acid-base experiments, strong acids or bases are typically chosen as reagents to ensure a clear endpoint, which is vital for accurate analysis. Additionally, the compatibility of the analyte with the reagent plays a significant role, as it facilitates a complete reaction, thereby minimizing the risk of incomplete titration and erroneous results.
In pharmaceutical laboratories, the choice between titrant vs titrand can markedly affect the measurement of active ingredient levels in medications. A pertinent case study illustrated how the improper selection of an indicator resulted in the misinterpretation of the endpoint, leading to a 10% overestimation of acetic acid concentration in a vinegar solution. This example underscores the necessity of comprehending the chemical properties of both components to avert inaccuracies in measurements.
Furthermore, the availability of suitable indicators that can effectively signal the endpoint is another critical consideration. Indicators change color at specific pH levels, such as phenolphthalein, which is commonly employed in acid-base analyses. However, the selection of an indicator must correspond with the anticipated pH at the equivalence point to guarantee reliable results.
As Dr. Julia Y. Harper aptly stated, 'Selecting the right titrant vs titrand is not merely a procedural requirement; it is akin to choosing the appropriate tool for a craftsman—a well-chosen titrant vs titrand enhances the quality of the outcome.' By meticulously evaluating these factors, researchers can enhance the reliability and accuracy of their results, ultimately bolstering the integrity of their analytical processes.
Considerations for Titration Techniques: Best Practices and Common Pitfalls
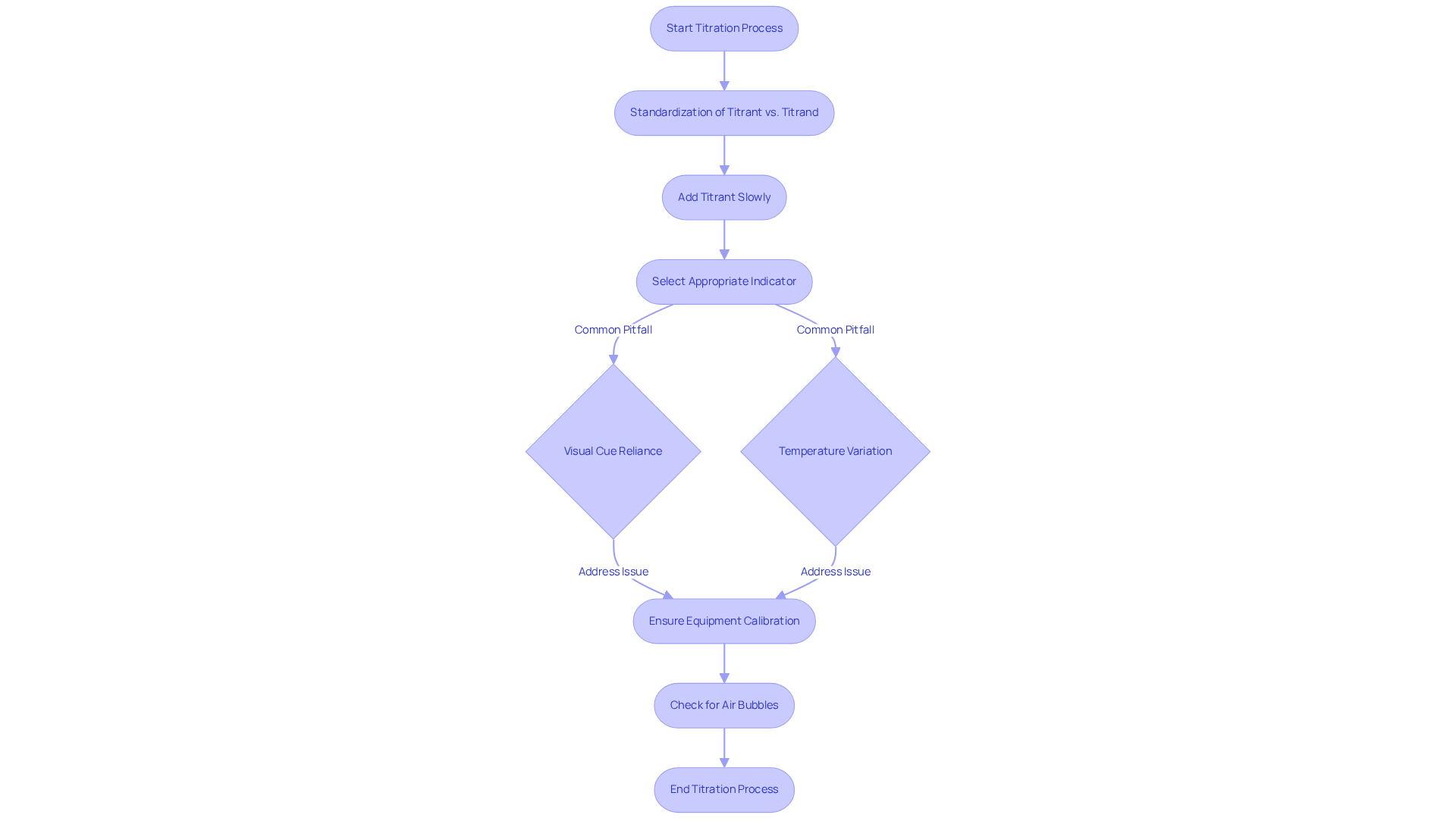
Effective measurement demands meticulous attention to detail and strict adherence to established best practices. A critical first step is the proper standardization of the titrant vs titrand; inaccuracies in concentration can lead to significant errors in results. To ensure reliability, the process of titrant vs titrand standardization is typically performed in triplicate at a minimum. Moreover, in the context of titrant vs titrand, titrants must be added slowly and carefully to prevent overshooting the endpoint, a common mistake that can skew results.
Common pitfalls in the process include the selection of inappropriate indicators, which can mislead the interpretation of the endpoint. For instance, reliance on visual cues such as phenolphthalein may result in inaccuracies due to personal color perception. Erroneous endpoint identification is a frequent problem in the process, often stemming from visual assessment techniques. Furthermore, neglecting to account for temperature variations can affect reaction rates, leading to inconsistent results. While most laboratories operate at room temperature, sensitive measurements may necessitate a water bath to ensure a stable temperature, as even minor variations can impact the precision of measurement results.
To mitigate these issues, laboratory professionals should implement best practices such as:
- Regular calibration of pH electrodes
- Ensuring that all equipment is clean and functioning properly
For example, air bubbles in burettes can lead to inaccurate low readings, making it crucial to remove these bubbles before commencing the process. By remaining vigilant about these factors, laboratories can significantly enhance the reliability and accuracy of their titration analyses.
Conclusion
Understanding the distinctions between titrant and titrand is essential for anyone engaged in analytical chemistry. The titrant, a solution with known concentration, plays a crucial role in determining the strength of the unknown titrand, which contains the analyte being measured. This interplay between the two substances is foundational for accurate titration results and highlights the importance of selecting appropriate reagents and indicators to achieve reliable outcomes.
Key insights from this discussion underscore the necessity of proper standardization, the critical role of indicators, and the implications of choosing the right titrant and titrand for specific laboratory applications. Common pitfalls, such as overshooting the endpoint and selecting inappropriate indicators, can significantly affect measurement accuracy. By adhering to best practices and understanding the chemical properties involved, laboratory professionals can enhance the reliability of their titration analyses.
Ultimately, the successful execution of titration techniques hinges on a deep comprehension of both titrant and titrand dynamics. Researchers are encouraged to carefully evaluate their choices and methodologies, ensuring they are equipped with the right tools and knowledge to achieve precise and accurate results. This meticulous approach not only bolsters the integrity of individual analyses but also contributes to the broader reliability of scientific research in the field.




