Overview
The fundamental differences between titrant and titrate are pivotal in the titration process. The titrant, a solution of known concentration, is added to the titrate, which is the solution undergoing analysis for its unknown concentration. Understanding these distinctions is not merely academic; it is essential in pharmaceutical laboratories, where accurate drug formulation and quality control are paramount. The effectiveness of titration has a direct bearing on the safety and efficacy of pharmaceutical products, underscoring the necessity for precision in these procedures.
Introduction
In the realm of analytical chemistry, the precise interplay between titrants and titrates is pivotal. This is particularly true within the pharmaceutical industry, where accuracy can determine the safety and efficacy of medications. This article delves into the critical distinctions between these two essential components of titration, illuminating their respective roles in ensuring reliable measurements and quality control in drug formulation.
As the demand for precision intensifies, challenges inevitably arise in maintaining the integrity of these substances. How can laboratories navigate the complexities of their interactions to uphold regulatory standards? This inquiry underscores the importance of understanding these dynamics, as they are crucial for achieving excellence in pharmaceutical development.
Define Titrant and Titrate: Core Concepts
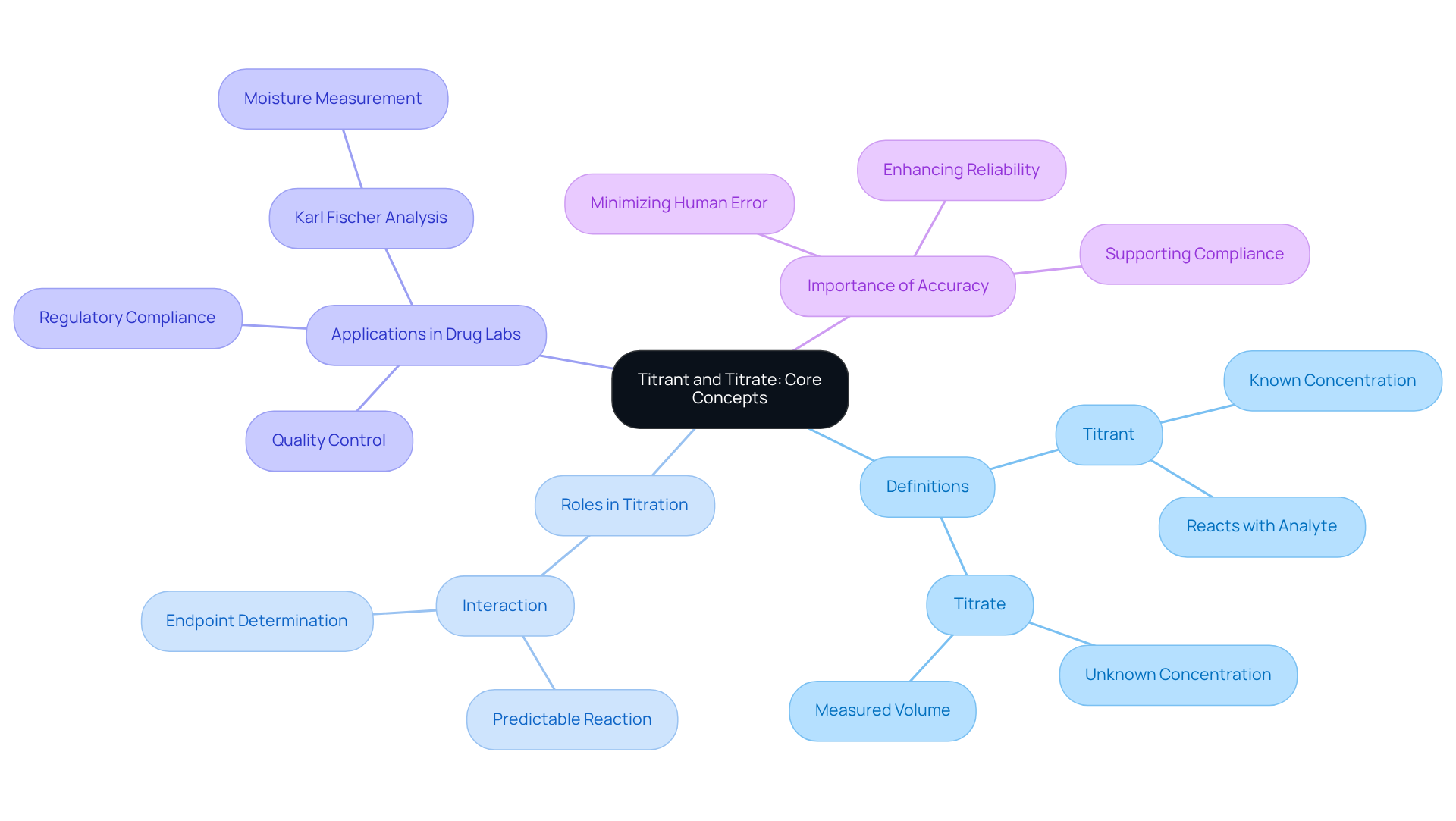
In analytical chemistry, the titrant vs titrate refers to a solution with a precisely known strength that is introduced to a solution of unknown strength during the titration process. This critical reagent reacts predictably with the analyte, facilitating the determination of the analyte's concentration. The significance of this process is particularly evident in drug laboratories, where precision is vital for quality control and adherence to regulatory standards. The term 'titrate' encompasses the analytical procedure of measuring the volume of titrant required to reach a specific endpoint in the reaction.
Understanding the distinct roles of titrant vs titrate is essential for effective titrimetric analysis, ensuring reliable results in drug formulation and quality assurance. For instance, in Karl Fischer analysis, the reagent interacts with water in a sample, enabling accurate assessment of moisture levels—an essential factor in the stability of medical products. Moreover, JM Science offers a variety of HPLC solutions and accessories that enhance method compatibility, significantly improving overall analytical capabilities.
The rising need for accurate measurement techniques in the drug industry underscores the importance of these roles. Furthermore, JM Science's automated measurement systems enhance accuracy and efficiency in laboratories, minimizing human error and supporting compliance with stringent industry regulations.
Examine Functions: Roles of Titrants and Titrates
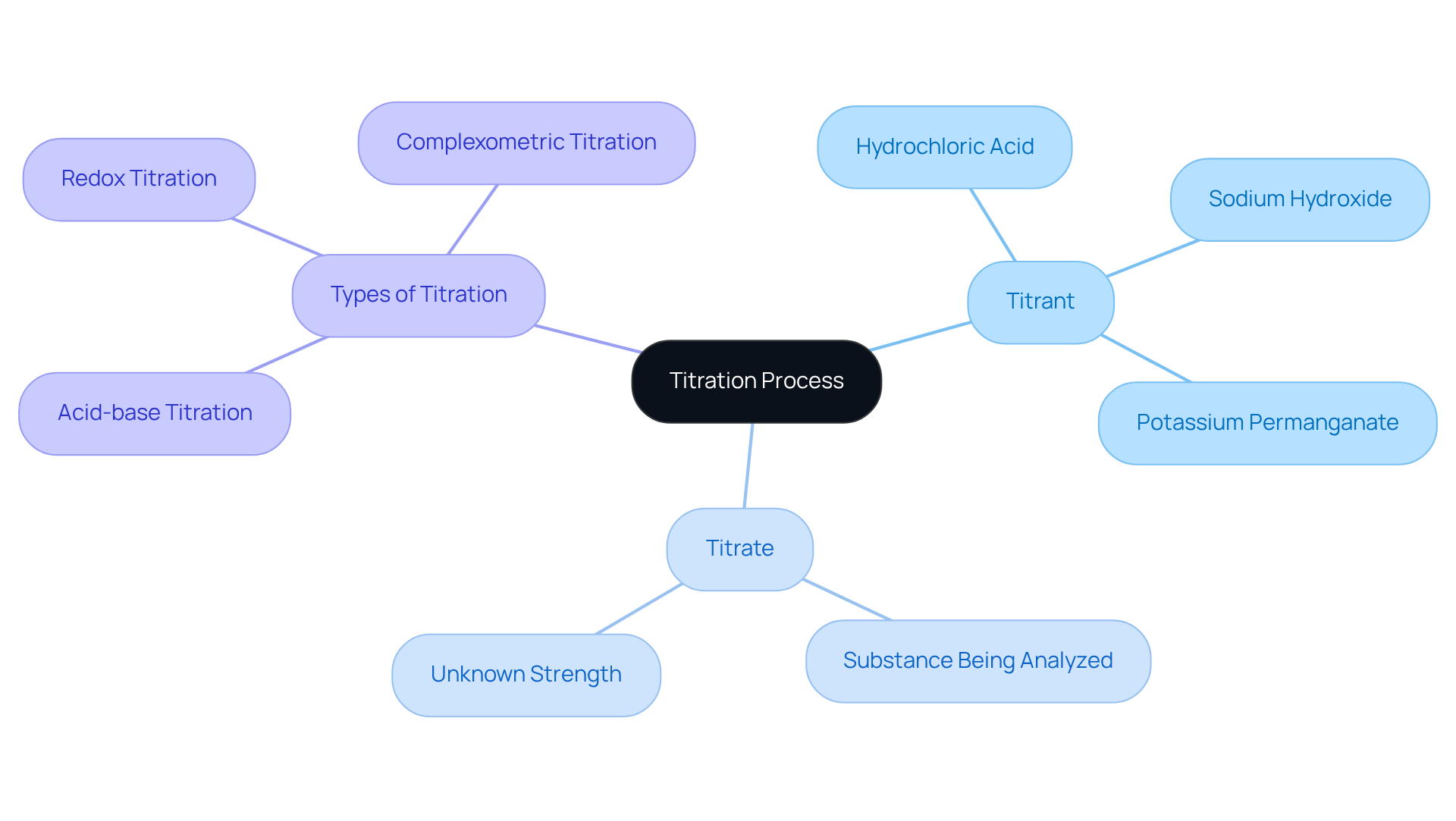
In pharmaceutical analysis, understanding the difference between titrant vs titrate is essential, as the titrant serves as the reactive agent added to the titrate, which is the substance being analyzed with an unknown strength. This critical procedure continues until the reaction reaches completion, typically indicated by a color change or a specific measurement. For example, in the context of titrant vs titrate, a strong acid may act as the titrant to ascertain the concentration of a weak base, which is the titrate. Such engagement is essential in drug laboratories, where accurate measurement techniques are paramount to ensuring the proper dosage of active components in medications.
JM Science Inc. offers a comprehensive range of premium titrators, HPLC columns, and accessories designed to enhance the accuracy and efficiency of these processes. Common reagents utilized in these settings include:
- Hydrochloric acid
- Sodium hydroxide
- Potassium permanganate
Each meticulously selected based on the specific requirements of the analysis. The effectiveness of this process in medical applications lies in its ability to deliver precise measurements of compounds, ensuring adherence to safety and efficacy standards. By mastering the dynamics of titrant vs titrate, industry professionals can significantly enhance the reliability of their analytical results, ultimately contributing to improved patient care.
Moreover, the selection of appropriate indicators is vital for achieving reliable analytical outcomes, as they signal the completion of the reaction. Titration techniques—including:
- Acid-base titration
- Redox titration
- Complexometric titration
are frequently employed in laboratory settings to guarantee quality control and precise measurement of active ingredients. As emphasized in the sector, "In medicine, precision is not optional; it is paramount.
Explore Applications: Titrants and Titrates in Practice
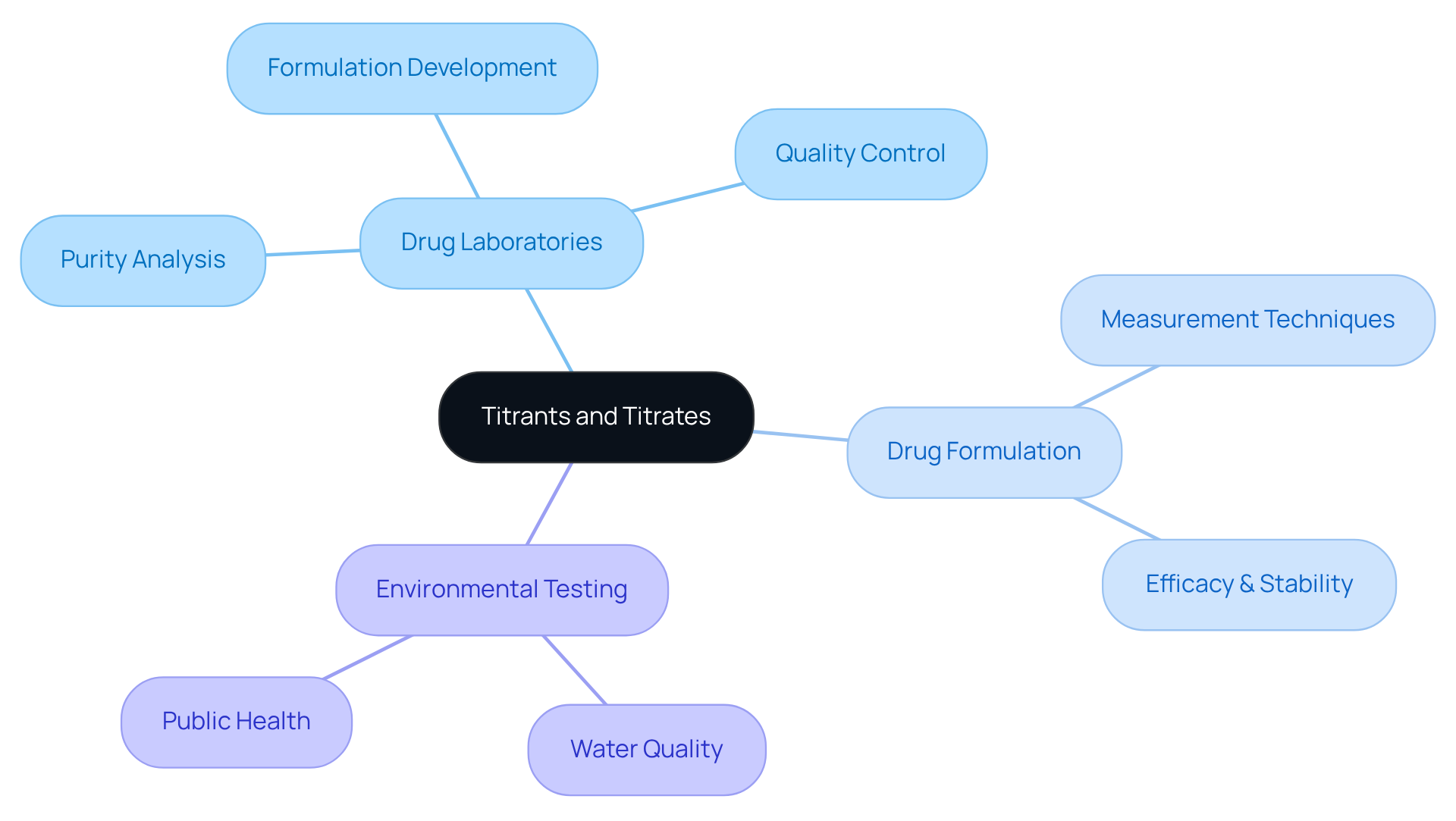
In drug laboratories, the roles of titrants vs titrate are indispensable, as they fulfill crucial functions such as drug purity analysis, formulation development, and quality control. This standard technique is essential for assessing the concentration of active medicinal components (APIs) in formulations, thereby ensuring compliance with stringent regulatory standards. The integrity and safety of pharmaceutical products hinge on this process.
In drug formulation, precise measurement techniques are employed to optimize the balance of ingredients, which significantly impacts the efficacy and stability of the final product. Additionally, these substances play a vital role in environmental testing, assisting in the evaluation of water quality by measuring pollutant levels, thus contributing to public health and safety.
Current trends reveal an increasing reliance on automated titration systems that enhance accuracy and efficiency while reducing human error. This automation is particularly advantageous in high-throughput environments, where consistent quality control is essential. As the drug industry evolves, the adaptability of titrants vs titrate confirms their critical role in both research and industrial applications.
Compare Advantages and Limitations: Titrants vs. Titrates
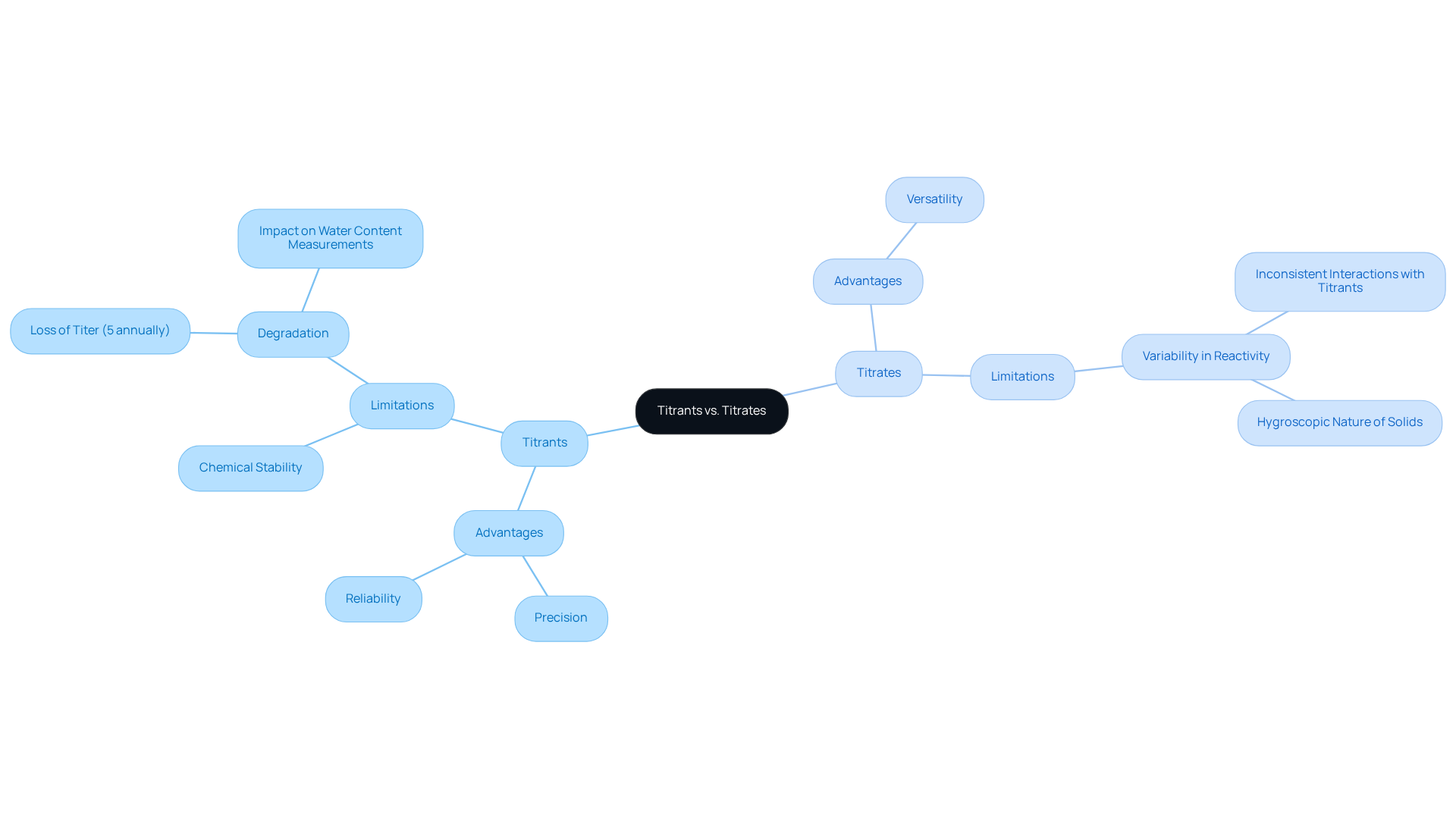
In comparing reagents, the distinctions between titrant vs titrate reveal distinct advantages and limitations. Standardized reagents are celebrated for their precision and reliability, making them indispensable for quantitative analysis in pharmaceutical environments. However, their effectiveness can be undermined by factors such as chemical stability and reactivity with other substances. For instance, single-component reagents, while simpler to use, require regular titer assessments due to their tendency to degrade over time, losing approximately 5% of their titer annually. This degradation can result in inaccuracies in water content measurements, particularly in sensitive applications. The decline in titer underscores the necessity for consistent checks on reagent integrity.
Conversely, titrates exhibit a diverse range of characteristics that can complicate measurement processes. Variability in reactivity may lead to inconsistent interactions with titrants, potentially skewing results. For example, hygroscopic solids, such as active pharmaceutical ingredients (APIs), can absorb moisture from the environment, causing inflated measurements during analysis. Implementing low-humidity environments and utilizing sealed titration cells can alleviate these concerns, ensuring more reliable outcomes. It is advisable to maintain relative humidity below 50% to reduce moisture interference.
Furthermore, the accuracy of standardized reagents is paramount for ensuring product safety and efficacy. Regular inspections of reagent integrity and appropriate storage conditions—such as maintaining temperatures between 4°C and 10°C and storing Karl Fischer reagents in tightly sealed containers to prevent moisture ingress—are essential for sustaining analyte stability. Expert insights emphasize that achieving precise outcomes in the process requires meticulous management of environmental factors, including pH and temperature, which can significantly influence reaction kinetics and endpoint identification. As Antoine Lavoisier aptly stated, "Accuracy is the essence of science."
In conclusion, understanding the limitations of both titrant vs titrate is crucial for pharmaceutical laboratories. By addressing these challenges through best practices and advanced titration systems, laboratories can enhance the accuracy and reliability of their analytical results, ultimately contributing to improved product quality and compliance with regulatory standards.
Conclusion
Understanding the distinctions between titrant and titrate is fundamental in pharmaceutical analysis. The titrant, with its known concentration, plays a pivotal role in determining the concentration of the unknown titrate. This interaction is crucial for ensuring precision and compliance with industry standards, ultimately safeguarding product quality and efficacy in the pharmaceutical sector.
Key insights have been highlighted throughout the article, including the specific functions of titrants and titrates in various analytical techniques such as acid-base and redox titrations. The importance of selecting appropriate reagents and indicators, as well as maintaining optimal laboratory conditions, has also been emphasized. Moreover, the advantages and limitations of these substances underscore the necessity for rigorous quality control and the adoption of advanced titration systems to enhance accuracy.
In a broader context, the meticulous management of titration processes is not merely an academic exercise; it is a vital component of pharmaceutical safety and effectiveness. As the industry continues to evolve, embracing automation and improved methodologies will be essential in meeting the growing demands for precision in drug formulation and quality assurance. By prioritizing a thorough understanding of titrant and titrate dynamics, pharmaceutical laboratories can significantly contribute to better health outcomes and regulatory compliance.




