Overview
The article examines the methods and procedures involved in titration, a fundamental quantitative analytical technique essential for determining the concentration of an unknown solution via a reaction with a known reagent. It underscores the critical importance of precise measurement and explores various titration types, including acid-base and redox reactions. By adhering to meticulous procedures, one can ensure reliable results, thereby affirming titration's pivotal role in crucial fields such as pharmaceuticals and environmental monitoring. This comprehensive overview highlights the necessity for accuracy and reliability in scientific practices, urging professionals to prioritize high-quality methods in their laboratory work.
Introduction
Understanding the intricacies of titration is essential for anyone involved in analytical chemistry, where precision can mean the difference between success and failure. This tutorial delves into the fundamental principles, methods, and procedures of titration, offering readers a comprehensive guide to mastering this critical technique.
As advancements in technology reshape the landscape of analytical practices, professionals must ensure they are utilizing the most effective methods while avoiding common pitfalls. By grasping these concepts, one can navigate the complexities of titration with confidence and accuracy.
Define Titration: Principles and Importance
A titration example is a quantitative analytical method used to determine the amount of a substance in an unknown solution (the analyte) by reacting it with a solution of known strength (the reagent). This method necessitates the meticulous addition of the reagent to the analyte until the reaction reaches its endpoint, typically indicated by a color change or a measurable alteration in a physical property. The process is fundamentally rooted in the stoichiometric relationship between the reactants, enabling precise computations of density based on the volume of reagent utilized.
In 2025, the current trends in analytical techniques underscore the integration of advanced technologies, such as potentiometric measurements and smartphone applications, which enhance accuracy and accessibility for users. These innovations are particularly beneficial in pharmaceutical research, where precise measurement is crucial for quality control and regulatory compliance, ensuring that active ingredients meet specified concentrations. For example, a smartphone-based pH reading application has demonstrated results that align with those obtained from a standard pH meter, illustrating the transformative impact of technology on analytical practices.
The significance of this analytical process, exemplified by a titration example, extends beyond mere measurement; it serves as a foundational technique in analytical chemistry, providing reliable and reproducible results essential for research and quality assurance. Specialists in the field emphasize the importance of accuracy in this process. A chemist once stated, "The endpoint of a measurement is a signal; the equivalence point is the reality," highlighting the critical nature of precise endpoint assessment. Additionally, another chemist noted, "Data visualization converts raw figures into insight; the curve narrates a tale beyond simple computations," underscoring the necessity of understanding these curves in relation to analytical outcomes.
Furthermore, the real-world applications of this analytical technique are extensive, spanning from environmental monitoring to food and beverage testing, where it assesses acidity levels and ensures adherence to health standards. A case study on measuring titratable acidity in wine serves as a titration example that highlights the practical applications of analytical techniques within the food and beverage sector. By mastering measurement techniques, chemists enhance their analytical skills, significantly contributing to scientific inquiry and advancements across various industries. Regular calibration of instruments is also essential to maintain precision and reliability in results, further emphasizing the method's role in ensuring quality control.
Explore Types of Titration: Acid-Base, Redox, and More
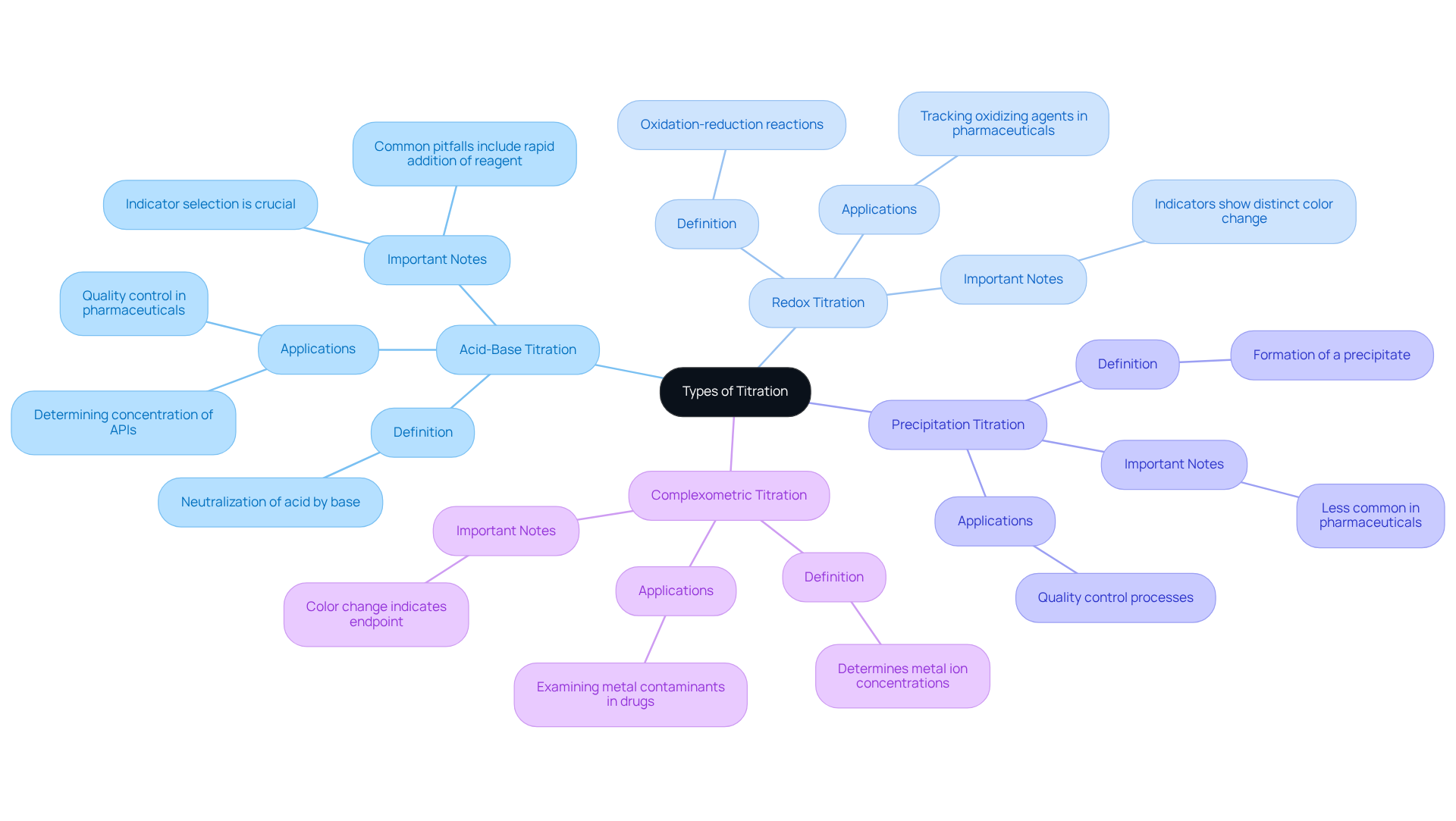
A titration example demonstrates how titration methods are essential analytical techniques, each meticulously tailored for specific chemical reactions. The primary types include:
-
Acid-Base Titration: This widely used method involves the neutralization of an acid by a base, with the endpoint typically indicated by a color change in a pH indicator. Acid-base titrations have historical roots dating back to the mid-1800s, with contributions from Karl Friedrich Mohr. They are crucial in pharmaceutical labs for determining the concentration of Active Pharmaceutical Ingredients (APIs) and ensuring product quality. However, common pitfalls such as rapid addition of reagent and inappropriate indicator selection can lead to inaccuracies, emphasizing the need for careful technique.
-
Redox Titration: This technique focuses on oxidation-reduction reactions, where the titrant alters the oxidation state of the analyte. Indicators in redox analyses often show a distinct color change at the endpoint. Present trends in redox analysis applications involve tracking the levels of oxidizing agents in pharmaceutical formulations, which is essential for ensuring drug efficacy and safety.
-
Precipitation Titration: In this method, a precipitate forms during the reaction, and the endpoint is reached when no further precipitate appears. Although this technique is less common in pharmaceuticals, it can be applied in quality control processes.
-
Complexometric Titration: This method determines metal ion concentrations by forming a complex with a chelating agent. The endpoint is indicated by a color change due to the formation of a colored complex. Complexometric analyses are particularly useful in pharmaceutical labs for examining metal contaminants in drug formulations.
As pointed out by leading chemists, the selection of the measurement technique is essential for precise results. One chemist noted, "The selection of the indicator is as crucial as the selection of the titrant," underscoring the importance of choosing suitable indicators for each type of analysis. Moreover, statistical evaluations can be employed to assess the reliability of data from repeated experiments, reinforcing the significance of accuracy in measuring processes. Understanding these methods and their applications, including a titration example, is vital for pharmaceutical professionals aiming to ensure precision and compliance in their analytical processes.
Conduct Titration: Step-by-Step Procedure
To conduct a titration effectively, follow these essential steps:
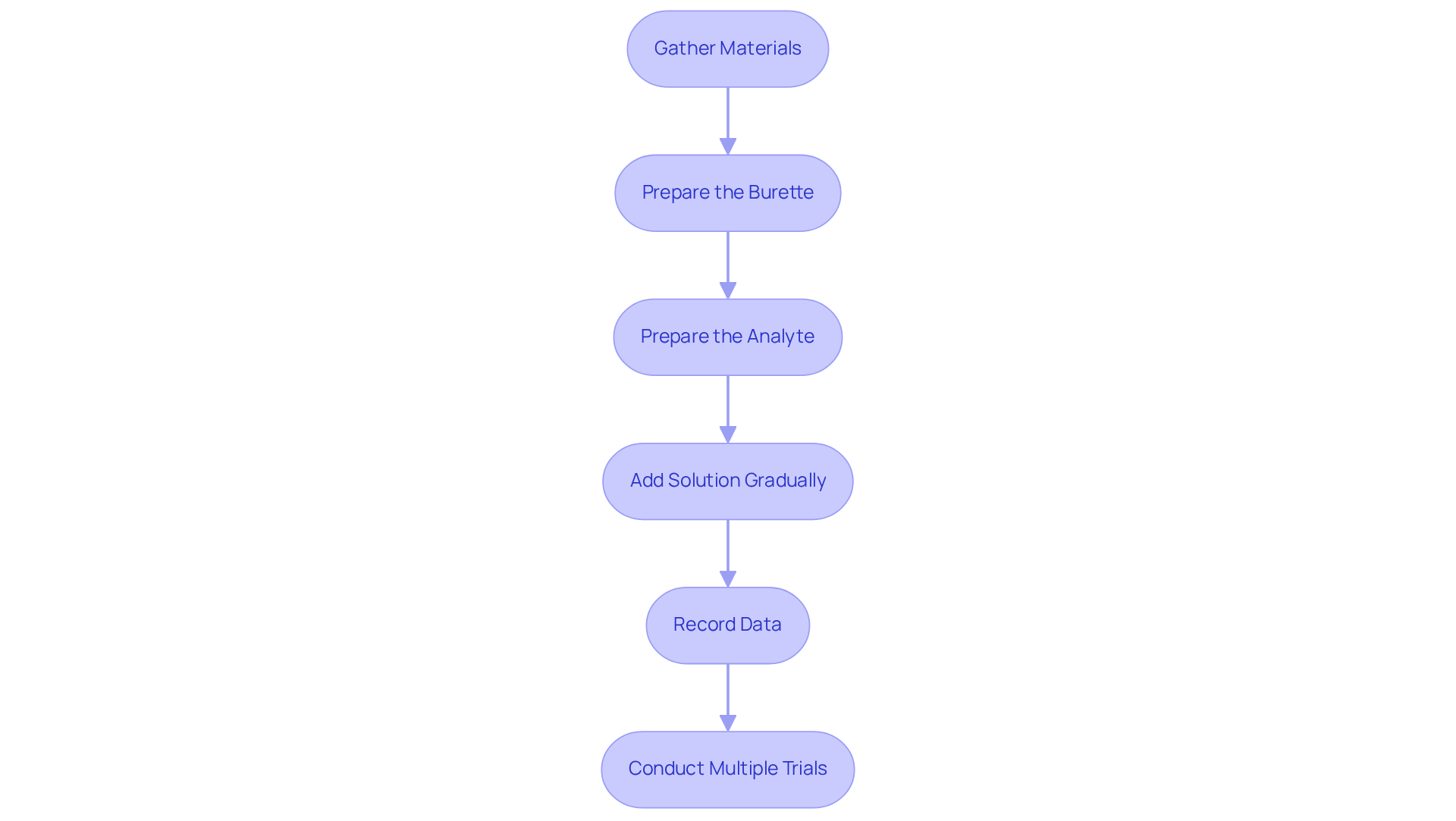
- Gather Materials: Key items include a burette, pipette, Erlenmeyer flask, reagent, analyte, and an appropriate indicator.
- Prepare the Burette: Clean the burette thoroughly with distilled water, then rinse it with the solution to prevent contamination. Fill the burette with the solution and note the starting amount.
- Prepare the Analyte: Use a pipette to transfer a measured amount of the analyte solution into the Erlenmeyer flask. Add a few drops of the selected indicator.
- Gradually add the solution from the burette to the analyte while continuously swirling the flask. Monitor for the endpoint, indicated by a distinct color change.
- Record Data: Upon reaching the endpoint, note the final amount of the reagent in the burette. Determine the amount of solution used by subtracting the starting quantity from the ending quantity.
- To enhance accuracy, conduct the procedure at least three times and compute the average volume of titrant used.
Implementing these steps serves as a titration example, ensuring that your analysis is conducted with precision and yielding reliable results. Optimal methods, such as maintaining a regulated environment and utilizing high-quality reagents, further enhance measurement accuracy. Laboratory managers emphasize the necessity of meticulous documentation and peer collaboration to minimize errors and validate findings. As noted by David Cash, PhD, "the percent by mass of total acid in individual Vicks brand sour hard sugar candies of the same batch was found to be considerably more variable than that in individual Sorbees sour hard sugar-free candies," underscoring the importance of careful monitoring of acid content. The average duration for conducting a quantitative analysis typically spans 30 to 60 minutes, influenced by the complexity of the process and the number of trials conducted. The titration example of Vicks and Sorbees candies serves as a case study that illustrates the practical applications of these methods and emphasizes the significance of precision in measurement.
Analyze Results: Calculations and Interpretation
Analyzing a titration example involves several critical calculations and interpretations that are essential for accurate chemical analysis.
-
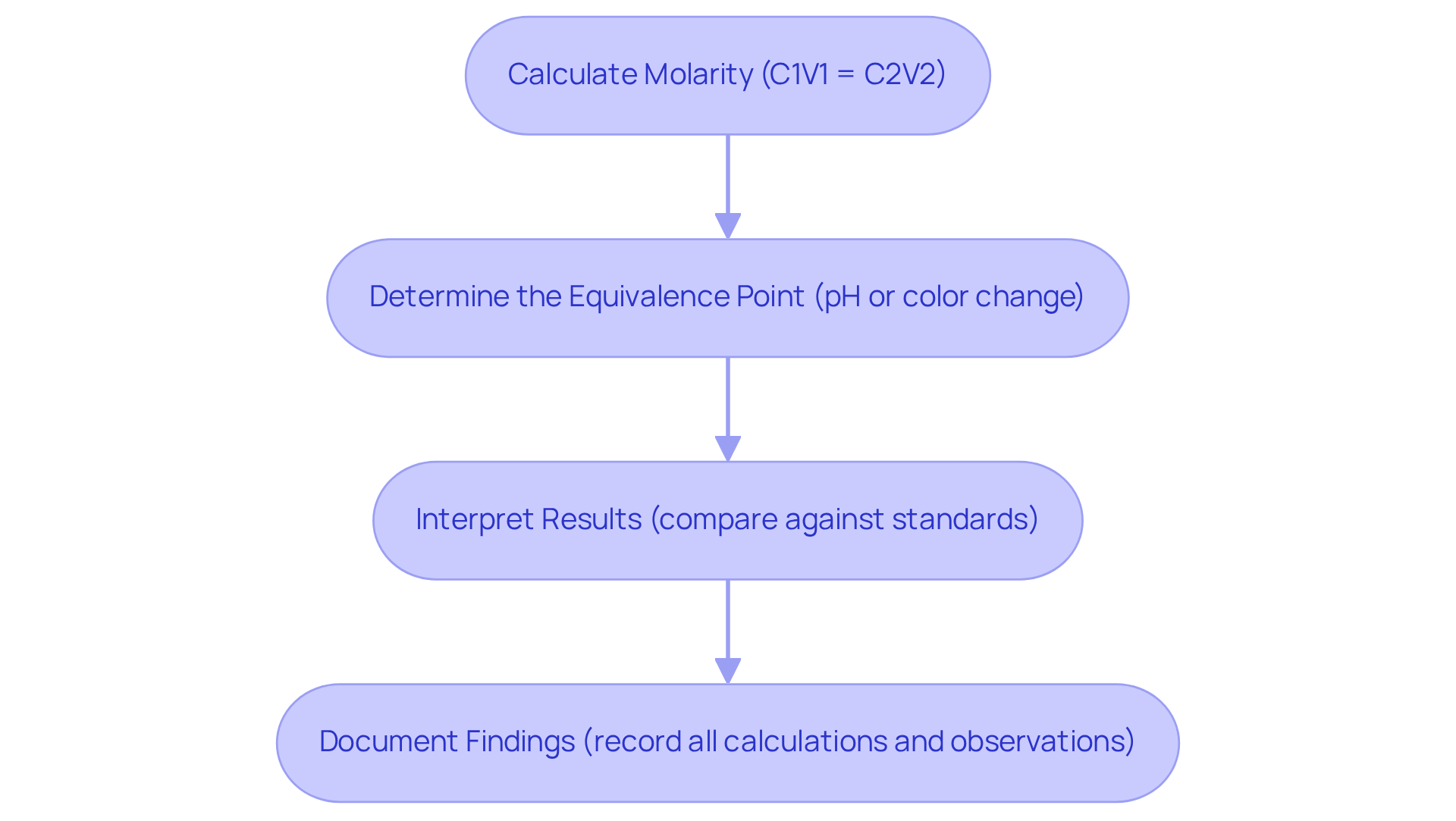
Calculate Molarity: Utilize the formula C1V1 = C2V2, where C1 signifies the concentration of the reagent, V1 is the amount of the reagent applied, C2 is the concentration of the analyte, and V2 is the amount of the analyte. For instance, if 9.88 mL of 0.200 M H3PO4 is fully neutralized by a specific volume of NaOH, the molarity can be derived from these values. Additionally, in a practical example, 40.50 mL of 0.0150 M aqueous HI is required to titrate 50.00 mL of an aqueous solution of NaOH, illustrating the application of this calculation.
-
Determine the Equivalence Point: This key point occurs when the amount of titrant added is stoichiometrically equivalent to the analyte present. It is typically identified by a noticeable change in pH or color, marking the transition in the reaction. For example, in a strong acid-strong base neutralization, the pH at the equivalence point is 7. As noted by prominent chemist Henry A. Berthelot, "The titration curve is a canvas on which the reaction’s story is painted," emphasizing the importance of understanding this concept.
-
Interpret Results: Compare the calculated level of the analyte against expected values or standards to evaluate accuracy. Discrepancies should prompt an investigation into potential sources of error, such as measurement inaccuracies or environmental factors that could affect pH readings. For instance, the titration example in the case study 'Role of Titrations in Determining Concentration of Unknown Solutions' highlights how careful interpretation can lead to accurate concentration values.
-
Document Findings: Meticulously record all calculations, observations, and interpretations in a lab notebook. This practice not only aids in future reference but also ensures compliance with laboratory standards, enhancing the reliability of your results. Systematic methods in recording findings can significantly reduce variability in measurement results, as highlighted in best practices.
By mastering these calculations and interpretations, you will significantly improve your analytical skills, contributing to more accurate and reliable laboratory outcomes. As noted by various chemists, understanding the intricacies of titration results is essential for effective chemical analysis and quality control in various applications, including pharmaceuticals and environmental monitoring.
Conclusion
Titration stands as a fundamental analytical technique, crucial for determining the concentration of unknown solutions through precise chemical reactions. By meticulously adding a reagent of known strength to an analyte until a defined endpoint is reached, this method guarantees accuracy and reliability across various scientific fields, particularly in pharmaceuticals and quality control.
This article highlights several key aspects of titration, including its principles, types, and step-by-step procedures. It delves into different titration methods, such as:
- Acid-base titrations
- Redox titrations
- Precipitation titrations
- Complexometric titrations
Emphasizing the importance of selecting appropriate indicators and techniques for accurate results, it outlines a systematic approach to conducting titrations, from gathering materials to analyzing results and documenting findings, reinforcing the necessity of precision in laboratory practices.
In light of significant advancements in analytical techniques and the integration of modern technologies, the mastery of titration cannot be overstated. As industries increasingly demand higher accuracy and reliability, professionals in the field must remain adept in their analytical skills. Embracing these methods not only enhances scientific inquiry but also ensures compliance with regulatory standards, ultimately contributing to advancements across various sectors. Engaging with titration practices and understanding their implications is essential for fostering innovation and maintaining quality in analytical chemistry.




