Overview
The article underscores the critical importance of the peroxide number in laboratory analysis, highlighting its essential role in evaluating the oxidation levels of fats and oils. This evaluation is paramount, as it directly influences product quality and safety. Elevated peroxide numbers serve as indicators of rancidity and potential health risks, necessitating a thorough understanding of their implications.
Furthermore, the article elaborates on various measurement techniques and the necessity of compliance with food safety regulations, ensuring the integrity of edible oils. By doing so, it not only informs but also empowers readers to appreciate the significance of accurate assessments in maintaining high-quality standards.
Introduction
In the realm of food safety and quality assurance, the peroxide number stands as a crucial indicator of the condition of fats and oils. This measurement quantifies the concentration of peroxide compounds, serving as an essential tool for assessing the freshness and safety of edible oils. Elevated peroxide values directly impact consumer health and product integrity, signaling potential rancidity and spoilage as oxidation progresses. Therefore, regular monitoring becomes imperative for compliance with safety regulations.
Various techniques, from traditional iodometric titration to modern spectrophotometric methods, exist to accurately measure this critical parameter. Understanding the implications of peroxide values not only aids in maintaining high standards in food quality but also informs effective decision-making in laboratory analysis, ensuring that consumers receive safe and nutritious products.
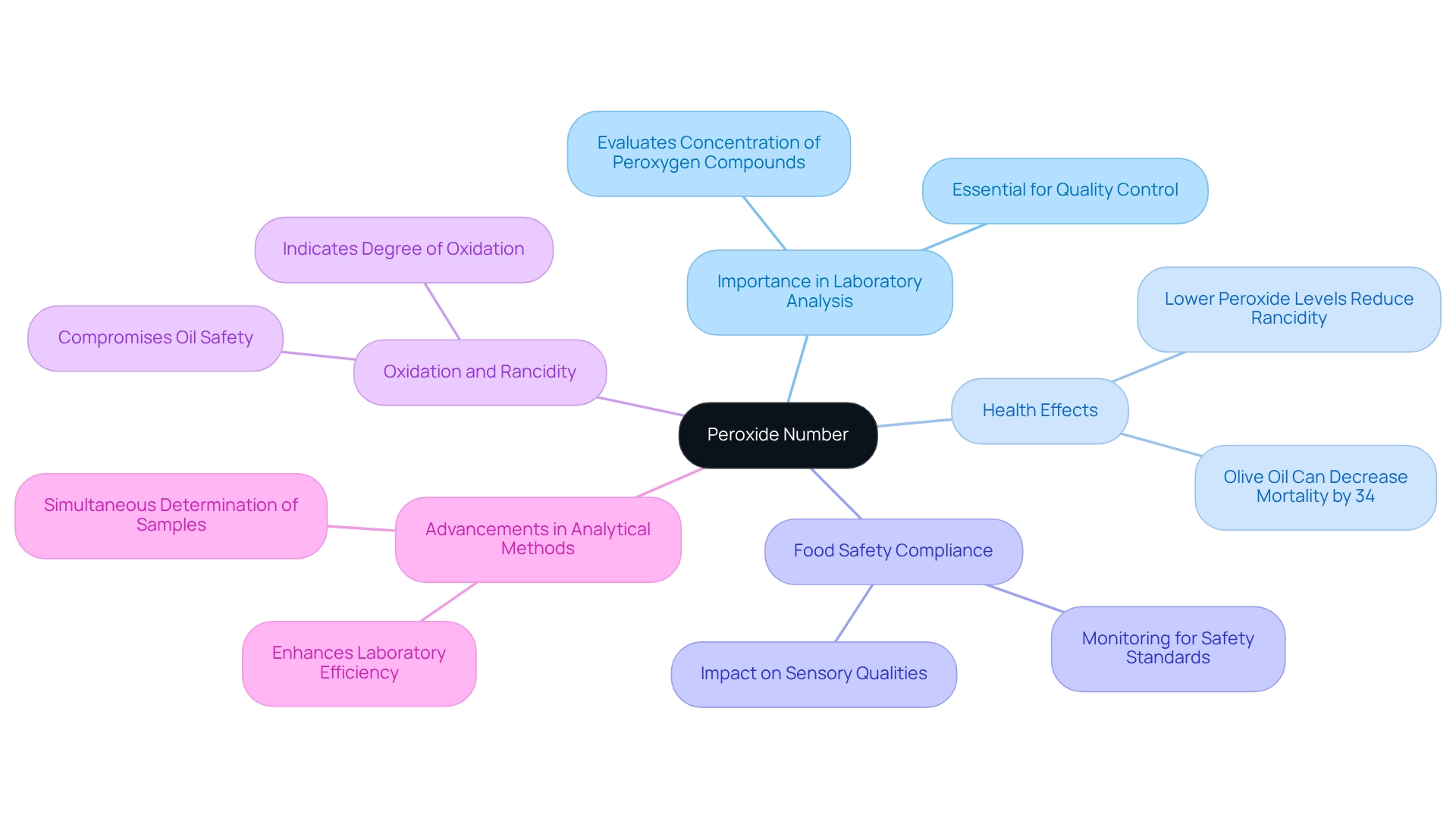
Define Peroxide Number and Its Importance in Laboratory Analysis
The peroxide number, commonly referred to as peroxygen measurement (PM), evaluates the concentration of peroxygen and hydroperoxide compounds in fats and oils, represented in milliequivalents of active oxygen per kilogram of fat. This metric is crucial as it reflects the degree of oxidation, which can lead to rancidity and spoilage. Elevated peroxide number levels indicate that an oil or fat has undergone considerable oxidation, potentially compromising its standard and safety.
Monitoring the peroxide number is essential for compliance with food safety regulations and for maintaining product integrity in laboratory environments. Frequent testing not only assists in evaluating the freshness of fats but also plays a vital role in monitoring the peroxide number throughout the food sector. Recent studies emphasize that measuring the peroxide number is a critical factor in lipid research and health evaluations of dietary fats, highlighting its significance in ensuring the safety and sensory qualities of oils. For instance, the case study titled 'Quantification of Peroxide Number (PN) in Oil' demonstrates how monitoring the peroxide number is essential for evaluating oil standards and safety, underscoring the necessity for thorough testing.
Studies suggest that olive oil, when adequately regulated for oxidation levels, can reduce mortality rates by as much as 34%. This statistic underscores the health effects of premium oils and emphasizes the importance of maintaining low oxidation levels. Furthermore, expert insights indicate that lower peroxide number and acid measurements correlate with a decreased probability of rancidity, reinforcing the importance of rigorous testing.
In practical applications, advancements in analytical methods now allow for the simultaneous determination of up to four samples, significantly enhancing laboratory efficiency. This innovation reinforces the continuous commitment to food safety adherence and assurance of standards, making the assessment of oxygenated compounds an essential element of laboratory analysis.
Explore Measurement Techniques for Peroxide Number
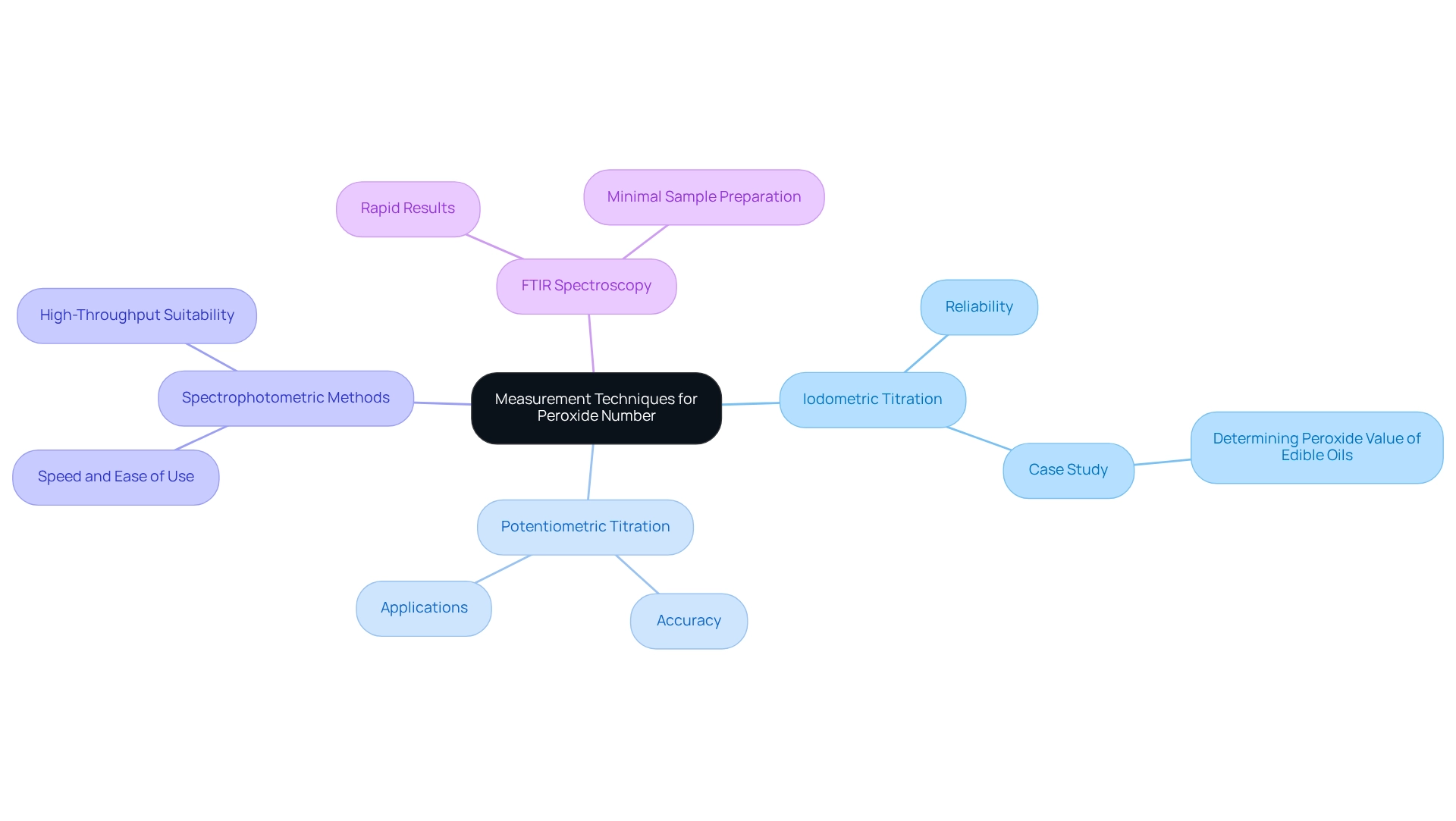
Several techniques are employed to measure peroxide number, each possessing distinct advantages and limitations.
Iodometric Titration stands out as a widely accepted method, involving the reaction of the sample with potassium iodide to liberate iodine, which is subsequently titrated with sodium thiosulfate. Renowned for its reliability, this method is the most commonly adopted approach in laboratories. A case study titled 'Determining Peroxide Number of Edible Oils' underscores iodometric titration as the predominant method, emphasizing its critical role in monitoring lipid oxidation and ensuring oil quality.
Potentiometric Titration employs an automatic titrator to measure potential changes in the solution, enabling precise determination of bleaching agent levels. Its accuracy renders it an invaluable option for laboratories requiring detailed analysis.
Spectrophotometric Methods measure the absorbance of a solution at specific wavelengths to ascertain compound concentration. Favored for their speed and ease of use, these methods are particularly suitable for high-throughput environments.
Fourier Transform Infrared (FTIR) Spectroscopy represents an advanced technique that provides rapid results without necessitating extensive sample preparation. By identifying compounds based on their distinct spectral signatures, FTIR serves as a contemporary substitute for measuring these substances.
The selection of measurement technique often depends on factors such as sample type, required sensitivity, and available laboratory equipment. Notably, recent research revealed mean concentrations after contact durations of 20, 30, and 40 minutes at 15.57, 7.57, and 6.58 mEq O2/kg, respectively. This data underscores the effectiveness of various measurement techniques. Additionally, Pradeep Kumar observed that the combination of metallized polypropylene packaging under nitrogen treatment optimally preserves walnut condition, further demonstrating the significance of precise measurement in maintaining oil integrity. As laboratories evolve, it is crucial to remain informed about the latest measurement methods and optimal practices for evaluating oxidation levels to ensure adherence to oil standards.
Analyze the Impact of Peroxide Number on Product Quality and Safety
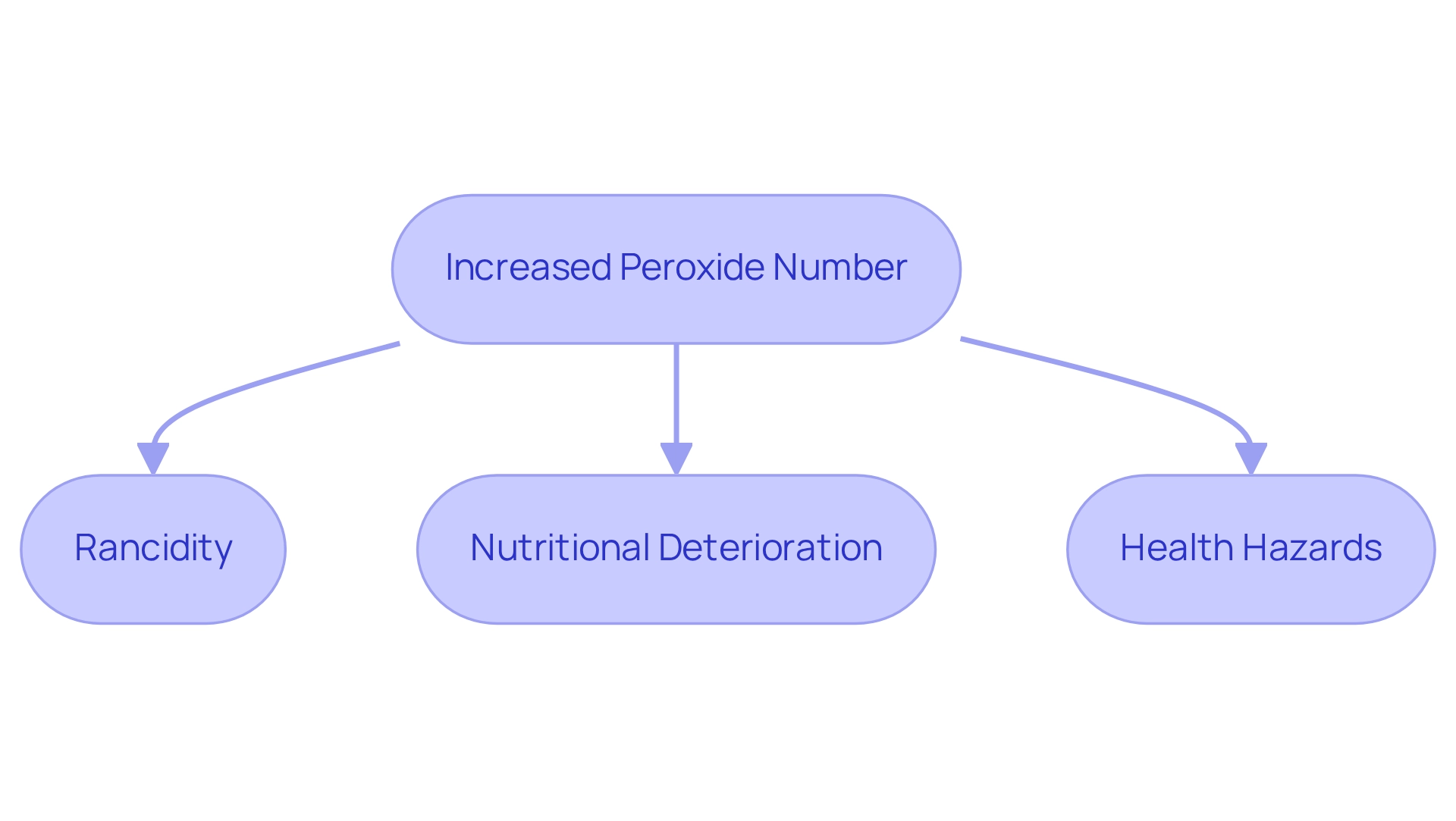
The peroxide number is crucial in evaluating the standard and safety of fats and greases. Elevated levels of oxidizing agents indicate advanced oxidation, leading to several detrimental effects:
- Rancidity: Increased levels contribute to rancidity, adversely affecting food's sensory qualities. This results in off-flavors and unpleasant odors, making the product unpalatable.
- Nutritional Deterioration: Oxidation diminishes the nutritional value of fats, leading to a reduction in essential fatty acids and vitamins necessary for health.
- Health Hazards: Consuming oxidized fats poses significant health risks, including the formation of harmful substances that may lead to chronic diseases.
Research indicates that the confidence level for inferential statistical analysis regarding the effects of oxidation levels, as measured by the peroxide number, on food quality is established at 95% (p < 0.05). This underscores the importance of regular assessments to ensure compliance with regulatory standards, which often specify the peroxide number for maximum allowable concentrations of edible fats. Such measures are vital for protecting consumer health and ensuring product safety.
Experts stress the necessity of monitoring the peroxide number and other chemical parameters to mitigate the adverse effects of rancidity. For instance, nutritionists note that increased viscosity in fats is a clear indicator of polymerization at advanced oxidation stages, complicating the assessment process. Understanding the relationship between viscosity and oxidizer concentrations is essential for grasping the peroxide number and oil stability. Additionally, case studies reveal challenges in collaborating with food suppliers to gather data on oil characteristics, indicating a need for improved engagement strategies in food safety research. These obstacles can impede the evaluation of bleaching agent values, such as the peroxide number, which are critical for ensuring oil quality. Furthermore, key observations regarding the physicochemical properties of discarded oils suggest no significant differences based on the frequency of oil filtration, indicating that oil stability may not be greatly influenced by filtration practices alone. This insight is vital for laboratories aiming to maintain oil standards over time.
By understanding the implications of oxidation levels, laboratories can implement effective control measures, ensuring high standards in product safety and compliance with health regulations. The ongoing need for further research on rapid testing methods for assessing frying oil standards highlights the essential role of the peroxide number in this context.
Interpret Peroxide Number Results for Effective Decision-Making
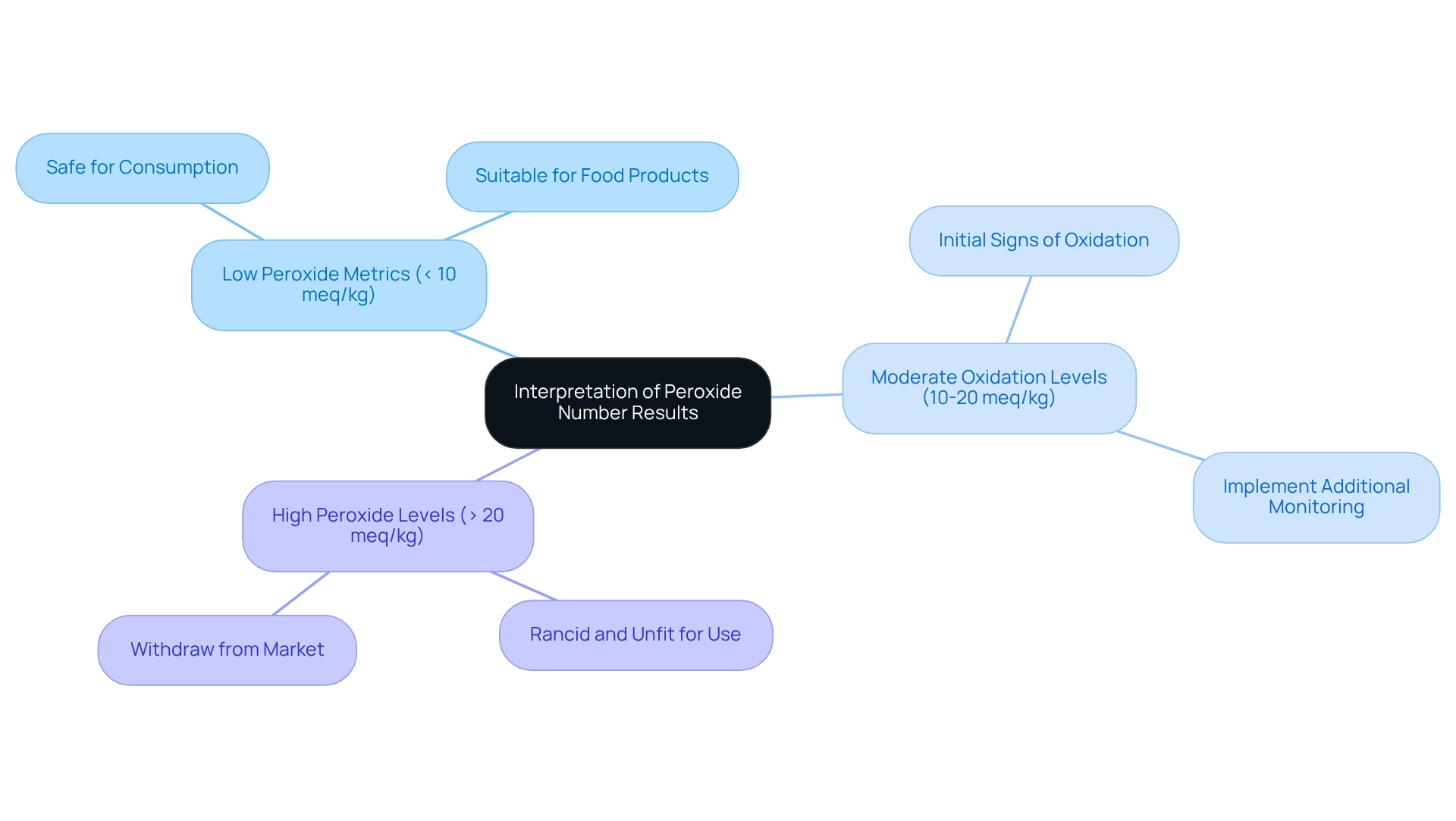
Analyzing the numerical results of the compound is essential for maintaining oil quality and safety. The subsequent classifications provide a framework for understanding these metrics:
- Low Peroxide Metrics (< 10 meq/kg): These figures indicate fresh oils with minimal oxidation, making them safe for consumption and suitable for food products. According to Codex standards, the oxidation level must remain below 10 meq O2/kg to ensure food safety.
- Oils exhibiting moderate oxidation levels (10-20 meq/kg) show initial signs of oxidation, as reflected in their peroxide number. While they may still be functional, it is advisable to implement additional monitoring to prevent spoilage and uphold product integrity.
- High peroxide number levels, specifically those exceeding 20 meq/kg, indicate significant oxidation, suggesting that the oil is rancid and unfit for use. Prompt measures, such as withdrawing the product from the market, are critical for safeguarding consumer health.
Laboratories should develop comprehensive procedures for addressing various oxidant number results. This includes:
- Retesting substances with moderate levels
- Discarding liquids with elevated oxidation rates
- Investigating potential issues related to storage conditions and handling methods
By adopting a proactive approach, laboratories can maintain product standards and adhere to safety regulations, ultimately protecting consumer health.
For instance, a study on the health risks associated with fried foods underscores the necessity of monitoring frying oil standards to mitigate health hazards, emphasizing that poor oil standards can lead to chronic diseases. This highlights the importance of routine testing of oxidizing substances in laboratory environments to ensure the safety and quality of fats used in food preparation. Furthermore, research indicates that the mean viscosity of discarded oils increases due to polymerization and oxidation, further illustrating the implications of high peroxide number values and the need for diligent monitoring. Additionally, samples were analyzed after 16 hours, with all measurements replicated three times for accuracy, reinforcing the reliability of the testing process.
Conclusion
The peroxide number stands as a crucial metric in evaluating fats and oils, reflecting their degree of oxidation and overall quality. It serves not only as an essential indicator for food safety but also plays a pivotal role in preventing rancidity and nutritional degradation. Regular monitoring of peroxide values ensures compliance with food safety regulations while preserving the integrity of products within the food industry. Various measurement techniques, such as iodometric titration and spectrophotometric methods, equip laboratories with the necessary tools to accurately assess peroxide levels, underscoring the importance of rigorous testing in quality control processes.
Grasping the implications of peroxide values is vital for informed decision-making concerning oil quality and safety. Low peroxide values signify fresh oils, whereas moderate values indicate early oxidation, necessitating additional monitoring. Conversely, high peroxide values point to significant spoilage, demanding immediate action to safeguard consumer health. The relationship between peroxide levels and sensory properties, nutritional value, and health risks emphasizes the urgent need for stringent testing protocols to ensure the safety of edible oils.
In summary, the peroxide number transcends mere laboratory measurement; it embodies a fundamental aspect of food quality assurance. By prioritizing regular testing and adopting best practices in measurement techniques, laboratories can protect consumer health and uphold high standards in food safety. As the food industry evolves, the importance of understanding and monitoring peroxide values will remain critical in delivering safe, nutritious, and high-quality products to consumers.




