Overview
The article examines the chemical structure of Polyethylene Terephthalate (PET) and underscores its significance across various applications, particularly within the food and beverage industry and sustainability initiatives.
PET's unique chemical composition, characterized by ester linkages and aromatic rings, enhances its durability and recyclability. These properties are essential for addressing the increasing demand for sustainable packaging solutions, making PET a pivotal material in contemporary environmental efforts.
Introduction
The remarkable versatility of Polyethylene Terephthalate (PET) positions it as a cornerstone of modern materials science, particularly in packaging and textiles. Its unique chemical structure not only boasts impressive durability and lightweight properties but also plays a crucial role in sustainability through recyclability. As the demand for eco-friendly solutions escalates, industries face the challenge of optimizing PET’s chemical composition and synthesis methods to meet both consumer needs and environmental goals. This exploration reveals an intricate balance between innovation and responsibility in the evolving landscape of PET applications.
Define Polyethylene Terephthalate (PET) and Its Importance
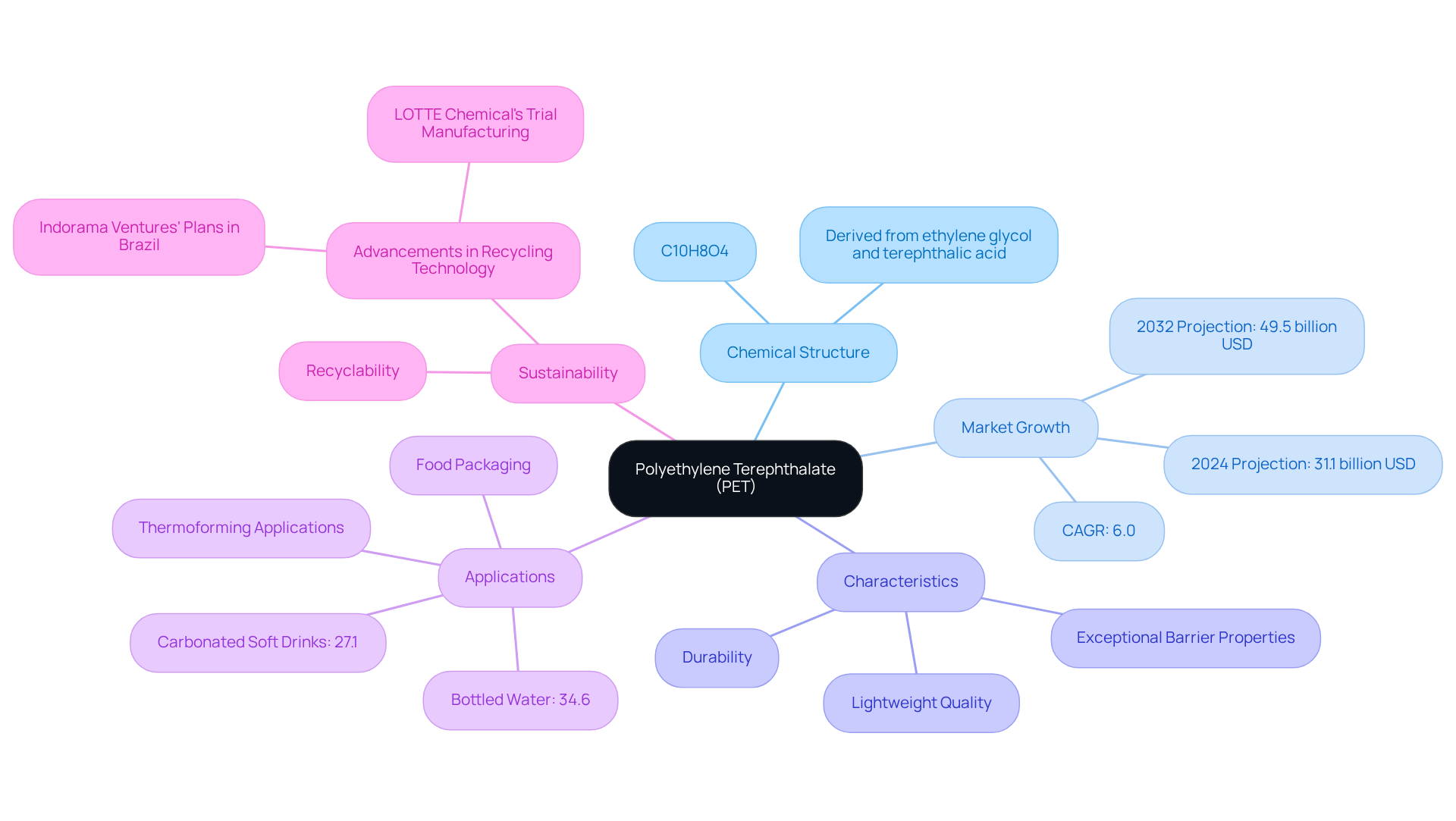
Polyethylene Terephthalate (PET) stands as a prominent thermoplastic polymer resin within the polyester family, extensively employed in fibers for clothing, liquid containers, and thermoforming applications. Characterized by the PET chemical structure represented by the formula (C10H8O4)n, PET consists of repeating units derived from ethylene glycol and terephthalic acid. Its notable characteristics—durability, lightweight quality, and exceptional barrier properties—render it particularly suitable for containment, especially in the food and beverage industry.
Looking ahead to 2024, the global market for PET is anticipated to reach approximately 31.1 billion USD, with projections indicating further growth to around 49.5 billion USD by 2032. This growth reflects a compound annual growth rate (CAGR) of 6.0% from 2025 to 2032, largely fueled by the increasing demand for sustainable container solutions. The recyclability of PET plays a pivotal role in sustainability initiatives, as it can be reprocessed into new products, significantly mitigating environmental impact. Recent advancements in recycling technology, such as Indorama Ventures' plans to enhance recycling capabilities in Brazil and LOTTE Chemical Corporation's trial manufacturing of recycled PET, underscore the sector's commitment to improving recycling processes.
The practical applications of PET within the food and beverage sector are extensive. Its significant roles in containers for carbonated soft drinks and bottled water accounted for 27.1% and 34.6% of global PET consumption, respectively, in 2019. The versatility of PET, combined with its affordability and durability, positions it as a leader in sustainable packaging solutions, effectively aligning with the increasing consumer demand for environmentally friendly products.
Explore the Chemical Structure of PET: Composition and Characteristics
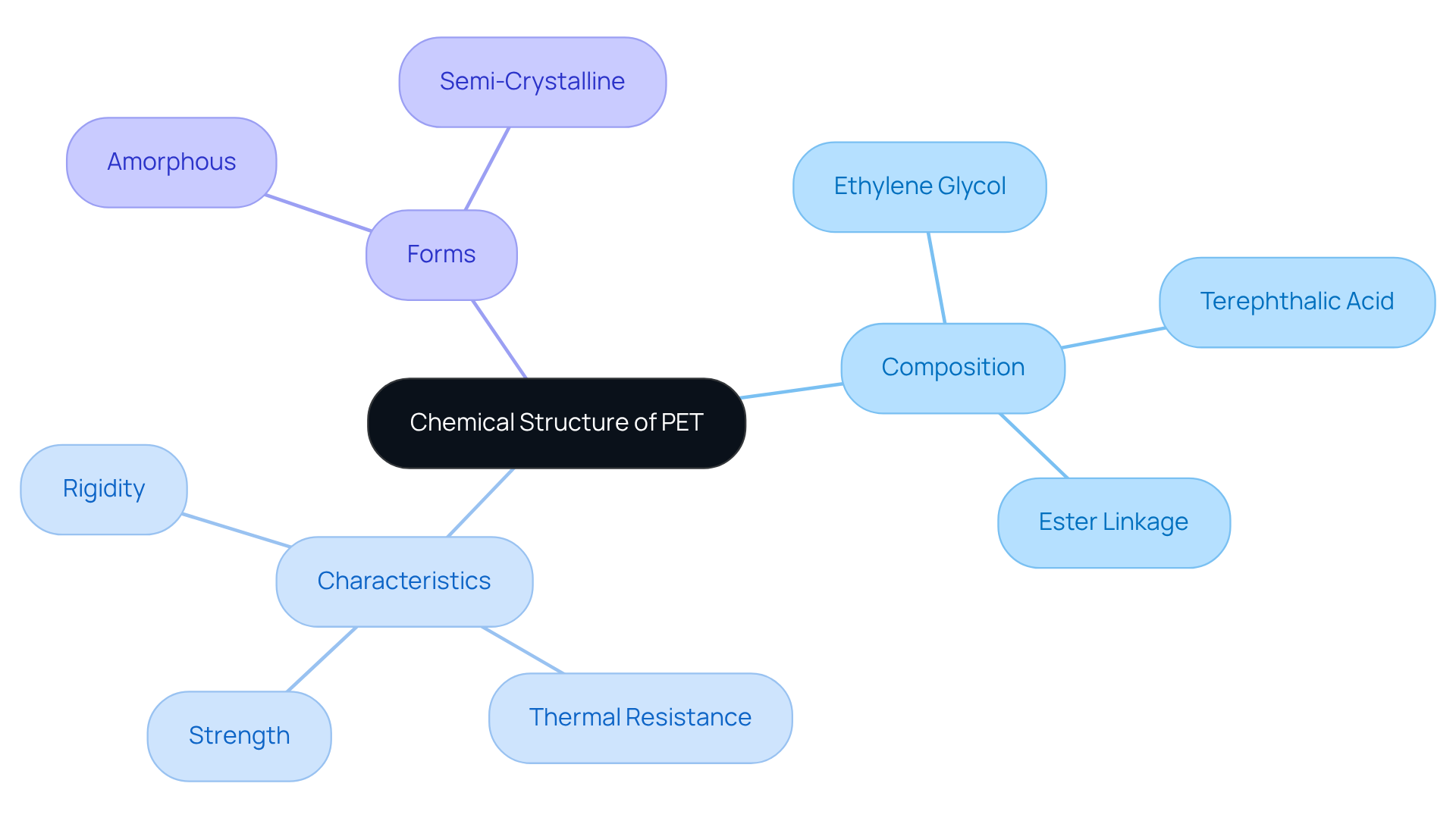
The pet chemical structure of PET is characterized by long chains formed through the polymerization of ethylene glycol and terephthalic acid. Each repeating unit features an ester linkage, which significantly contributes to the polymer's distinctive properties. Notably, PET can exist in both amorphous and semi-crystalline forms; the latter variant offers enhanced strength and thermal resistance. Additionally, the presence of aromatic rings within its structure increases rigidity and thermal stability, making it particularly suitable for high-performance applications. Understanding the pet chemical structure is crucial for predicting PET's behavior under varying conditions, such as temperature fluctuations and mechanical stress.
Examine the Synthesis Methods of PET: From Monomers to Polymerization
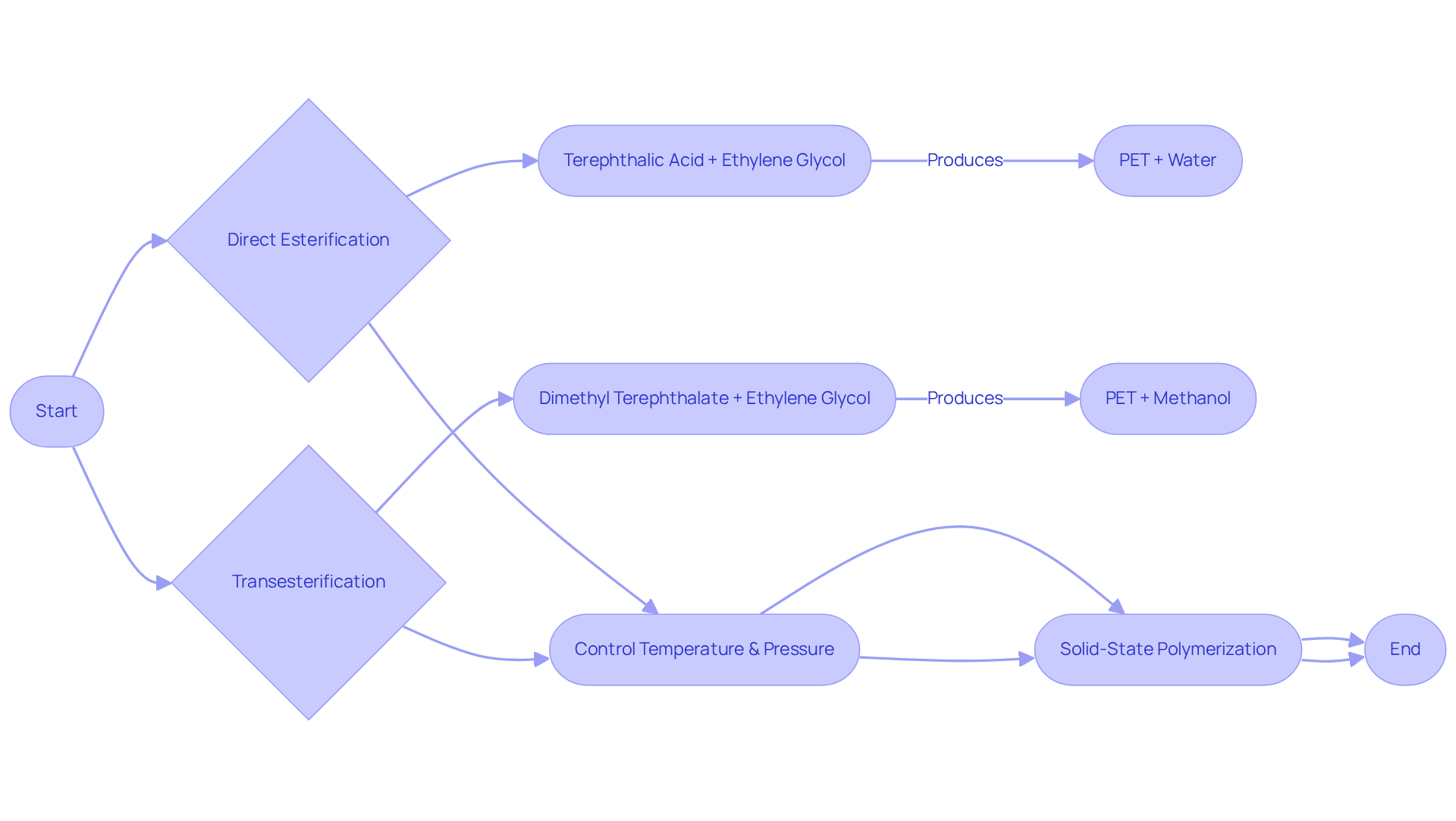
The synthesis of the pet chemical structure involves two primary methods: direct esterification and transesterification.
- In the direct esterification process, terephthalic acid reacts with ethylene glycol, resulting in the formation of a pet chemical structure and water as a byproduct.
- Alternatively, the transesterification process involves the reaction of dimethyl terephthalate with ethylene glycol, resulting in the formation of the pet chemical structure along with methanol.
Both methods necessitate meticulous control of temperature and pressure to achieve high yields and desired molecular weights. Furthermore, the polymerization process can be enhanced through solid-state polymerization, which increases the molecular weight of the polymer, thereby improving its mechanical properties. Understanding these synthesis methods is crucial for optimizing the pet chemical structure for specific applications in PET production.
Identify Applications of PET: Industries and Uses
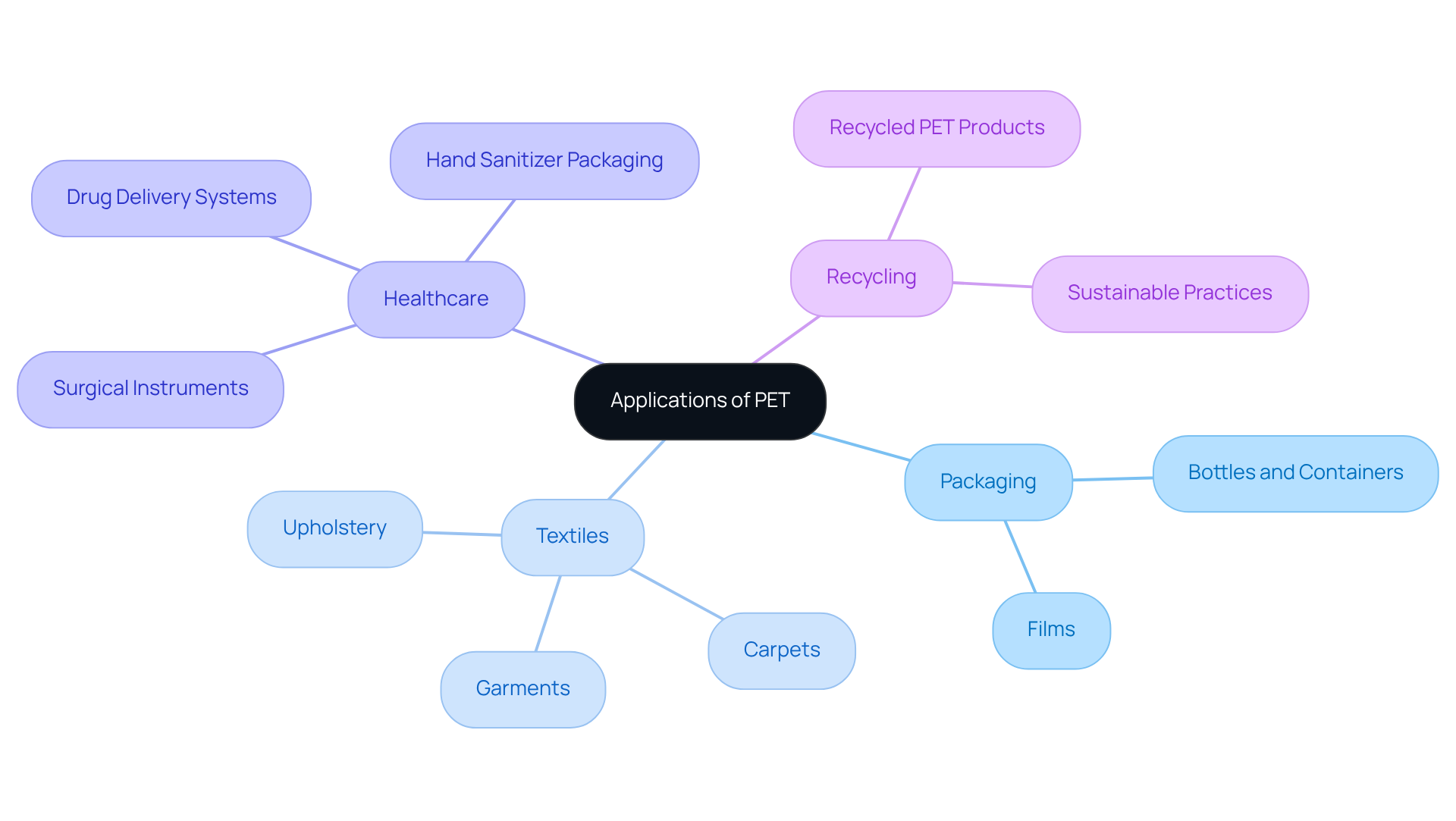
Polyethylene terephthalate (PET) is a versatile polymer whose beneficial properties are largely attributed to its pet chemical structure, making it extensively utilized across various industries. In the container sector, PET is the material of choice for manufacturing bottles, containers, and films, thanks to its outstanding barrier properties against moisture and gases. Recent statistics reveal that one million plastic bottles are purchased in the United States every minute, underscoring the high demand for PET in packaging applications.
Furthermore, the textile sector utilizes the pet chemical structure of PET fibers in garments, carpets, and upholstery, driven by an increasing need for durable and moisture-resistant materials, particularly in activewear and healthcare applications. Notably, the pet chemical structure's biocompatibility positions it as an ideal candidate for medical uses, including surgical instruments and drug delivery systems. The market for PET in healthcare is projected to reach USD 75.30 billion by 2025, growing at a compound annual growth rate (CAGR) of 5.71%.
Industry professionals assert that the adoption of PET in medical settings is pivotal for enhancing patient outcomes, especially in areas demanding precision and reliability. Additionally, the recycling of PET into recycled PET (rPET) has opened avenues for sustainable practices, allowing products to be created while maintaining the integrity of the pet chemical structure, including textiles and packaging materials.
Initiatives spearheaded by companies like LPLA and the Asahi Group exemplify the industry's commitment to sustainability, with LPLA's recyclable PET wine bottle achieving a 50% reduction in carbon footprints. This shift not only champions environmental sustainability but also resonates with the growing consumer awareness surrounding eco-friendly solutions. Understanding these applications reinforces the significance of the pet chemical structure in driving innovation and sustainability across multiple sectors.
Conclusion
In conclusion, Polyethylene Terephthalate (PET) stands out as a pivotal thermoplastic polymer, renowned for its extensive applications that span from packaging to textiles. This article underscores the critical importance of PET's chemical structure, which underpins its unique properties and facilitates its widespread utilization, particularly within the food and beverage sector. As the demand for sustainable materials intensifies, PET's role in eco-friendly solutions becomes increasingly vital, highlighting its significance in modern manufacturing practices.
Key insights reveal that PET's chemical composition—characterized by its repeating units of ethylene glycol and terephthalic acid—contributes to its durability, lightweight nature, and exceptional barrier properties. The synthesis methods, including direct esterification and transesterification, are essential for optimizing PET's molecular structure for specific applications. Furthermore, PET's versatility extends across various sectors, including healthcare and textiles, where its biocompatibility and durability prove invaluable.
Reflecting on broader implications, advancements in PET recycling technology and the commitment of companies to sustainable practices signal a transformative shift toward a more environmentally responsible future. As the PET market continues to expand, stakeholders across diverse industries are encouraged to adopt innovative strategies that leverage the unique properties of PET. This not only enhances product performance but also aligns with the growing consumer demand for sustainable solutions, establishing PET as a pivotal player in the journey toward a greener planet.




