Overview
This article delves into the critical understanding of error sources in titration, which can profoundly impact the accuracy of concentration calculations within laboratory environments. It underscores that errors may arise from:
- Human oversight
- Instrument calibration issues
- Environmental factors
Addressing these inaccuracies is paramount; thus, the article emphasizes the necessity of:
- Proper training
- The integration of advanced technology
- Strict adherence to standard operating procedures
These measures are essential for ensuring reliable analytical results and fostering a culture of precision in scientific inquiry.
Introduction
Understanding the intricacies of titration is crucial for lab managers. Even minor inaccuracies can lead to significant discrepancies in analytical results. By delving into various sources of error—ranging from human oversight to instrument calibration issues—laboratories can enhance the reliability of their findings. This ensures compliance with stringent research standards.
However, a pressing challenge remains: how can labs effectively identify and mitigate these errors while leveraging the latest technological advancements to improve accuracy?
Define Titration Errors and Their Importance
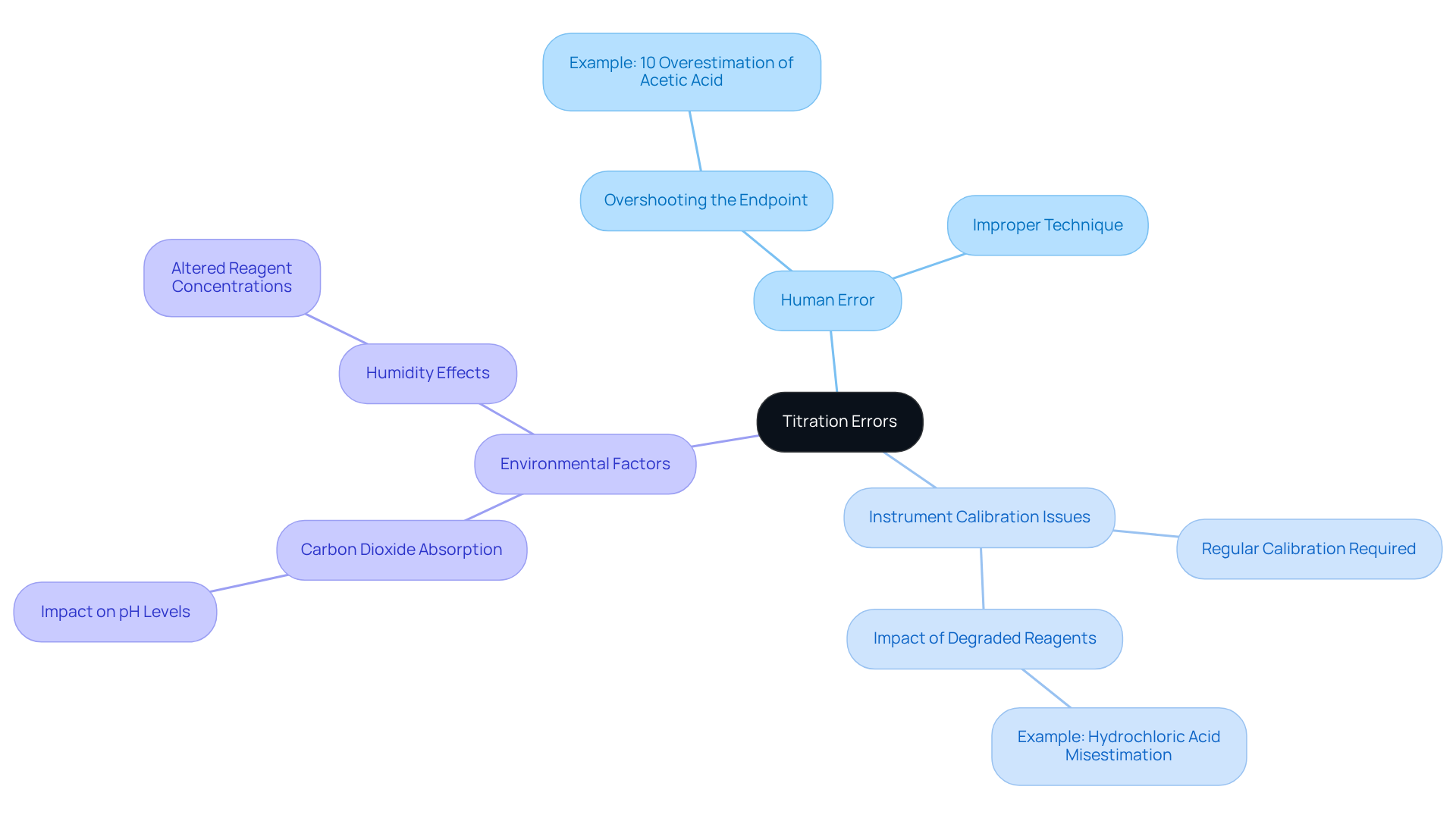
Titration inaccuracies are considered sources of error in titration that can occur during the process, leading to erroneous concentration calculations of the analyte. These inaccuracies arise from various sources of error in titration, including:
- Human error
- Instrument calibration issues
- Environmental factors
For example, a technician's oversight in a pharmaceutical lab resulted in a 10% overestimation of acetic acid concentration due to overshooting the endpoint. Understanding the sources of error in titration is vital for lab managers, as these mistakes significantly impact the reliability of analytical results, which in turn affects research outcomes and regulatory compliance. As Robert H. Grubbs aptly stated, 'The key to scientific discovery is the ability to identify and resolve mistakes.'
By acknowledging and addressing the sources of error in titration, laboratories can enhance the precision of their measurements, ensuring adherence to the stringent standards required in pharmaceutical and medical research environments. Conducting volumetric analyses in regulated settings is essential to mitigate external influences, such as carbon dioxide uptake, which can skew results. This commitment to minimizing errors not only fosters trust within the scientific community but also bolsters the validity of research findings, ultimately facilitating advancements in healthcare and pharmaceutical development.
Identify Systematic and Random Errors in Titration
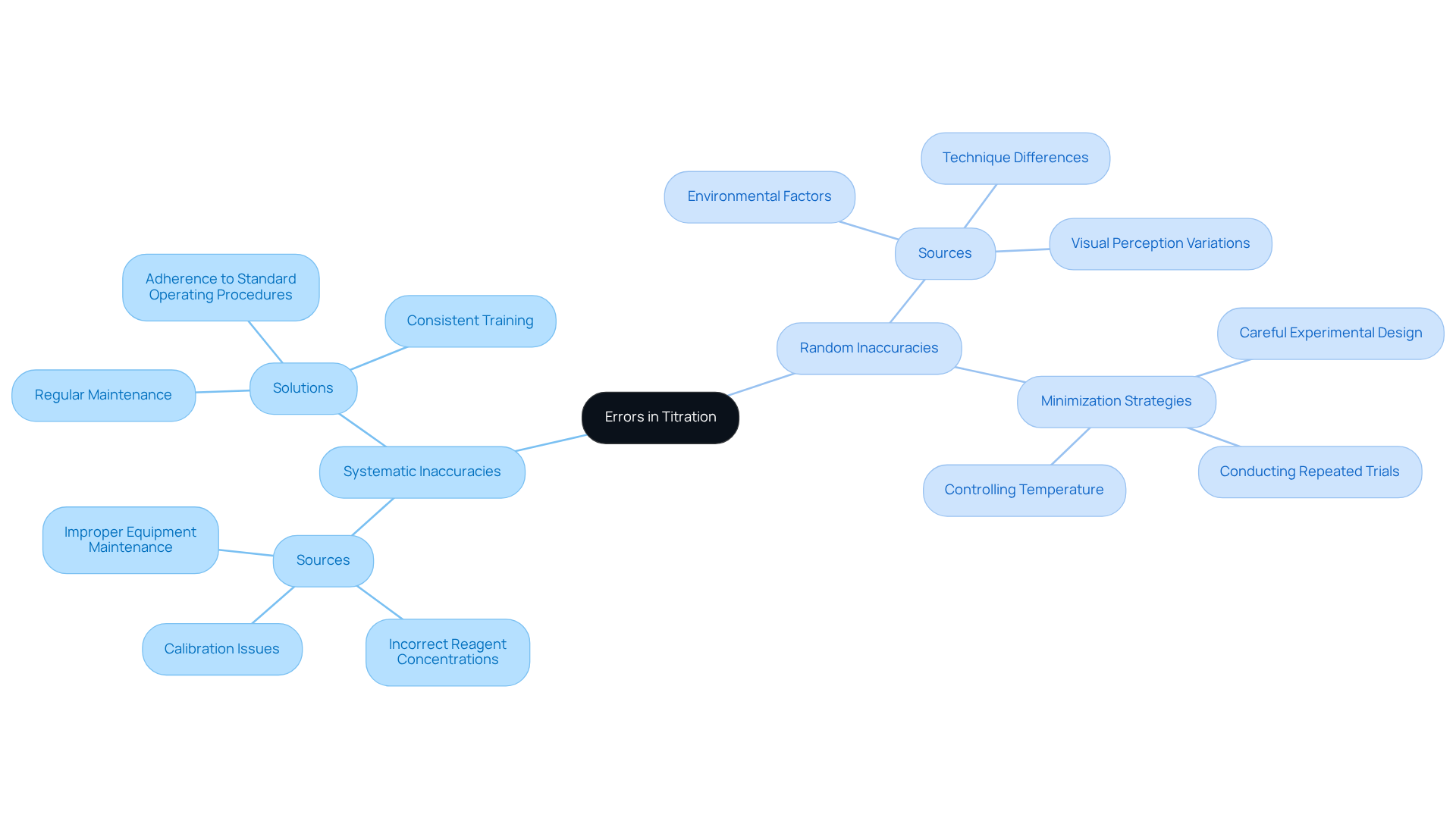
Sources of error in titration can be categorized into two primary types: systematic inaccuracies and random inaccuracies.
-
Systematic inaccuracies are considered sources of error in titration, referring to consistent errors stemming from flaws in the measurement process, such as improper calibration of instruments or incorrect reagent concentrations. These errors are often identifiable and can be rectified through regular maintenance and adherence to standard operating procedures.
-
In contrast, random inaccuracies are sources of error in titration that may arise due to unforeseen variations from environmental factors, such as temperature fluctuations or individual differences in technique. While it is impossible to eliminate random errors entirely, understanding the sources of error in titration can help minimize them through careful experimental design and repeated trials.
To effectively address systematic inaccuracies, lab supervisors should implement consistent training and calibration schedules, while also establishing procedures to account for random discrepancies in their analyses.
Leverage Technology to Enhance Titration Accuracy
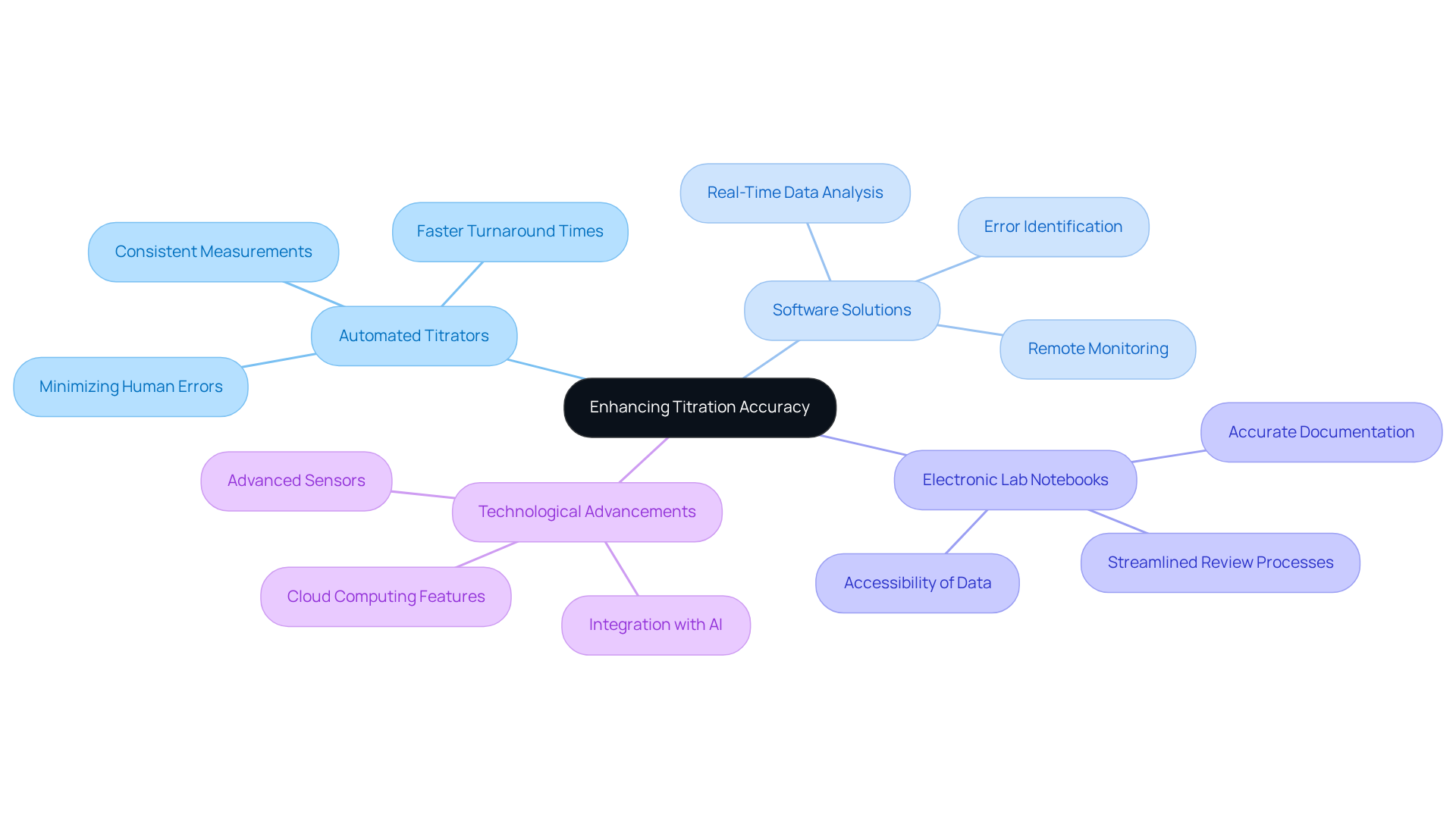
Incorporating technology into titration processes significantly enhances accuracy and efficiency. Automated titrators from JM Science, for instance, minimize human errors by precisely managing the addition of titrant and documenting findings in real-time. Moreover, software solutions can analyze data patterns, aiding lab managers in identifying potential errors before they impact results. The introduction of electronic lab notebooks further improves documentation, ensuring that all measurement procedures and outcomes are accurately recorded and readily accessible for review. By leveraging these technological advancements—including JM Science's innovative medical devices, Karl Fischer reagents, and HPLC solutions—laboratories can enhance the precision of their titrations and improve overall workflow efficiency, ultimately leading to more reliable research outcomes.
Conclusion
Recognizing the sources of error in titration is essential for lab managers dedicated to achieving precision in analytical results. By identifying the various inaccuracies that may arise—be it from human oversight, instrument calibration, or environmental influences—laboratories can markedly enhance the reliability of their measurements. This meticulous attention to detail transcends procedural necessity; it is foundational for preserving the integrity of research outcomes and ensuring compliance with regulatory standards.
The article underscores the critical distinction between systematic and random errors, highlighting the necessity of addressing both types to elevate titration accuracy. Systematic inaccuracies can typically be rectified through diligent calibration and strict adherence to established protocols. In contrast, random inaccuracies necessitate thoughtful experimental design and repeated trials to alleviate their effects. Moreover, leveraging technology, such as automated titrators and electronic lab notebooks, can further streamline processes and diminish the likelihood of human error.
Ultimately, a steadfast commitment to understanding and addressing titration errors is vital for propelling research in pharmaceuticals and healthcare. By cultivating a culture of precision and embracing the latest technological advancements, laboratories can not only bolster their analytical capabilities but also enhance the broader scientific community's trust in their findings. Proactively minimizing errors in titration ensures that research efforts yield meaningful advancements and reliable outcomes that benefit society as a whole.




