Overview
This article delineates the concepts of stationary phase and mobile phase in chromatography, elucidating their pivotal roles in the separation of components within a mixture. The interaction between these phases is not merely important; it is essential for effective separations, as demonstrated by various chromatography techniques. These techniques find extensive application in critical fields such as pharmaceuticals, environmental monitoring, and food safety, where precise separation and analysis are paramount for ensuring quality and compliance. Understanding these phases is fundamental for anyone involved in laboratory settings, emphasizing the necessity for high-quality scientific instruments.
Introduction
In the realm of scientific research, chromatography emerges as an indispensable technique, seamlessly connecting diverse disciplines through its remarkable ability to separate and analyze complex mixtures. The intricate interplay between stationary and mobile phases forms the foundation of this method, profoundly influencing critical areas such as pharmaceutical safety and environmental monitoring.
As advancements continue to propel the field forward, a comprehensive understanding of the principles and mechanisms of chromatography becomes essential for researchers eager to harness its full potential. This article investigates the fundamental aspects of chromatography, examining its principles, separation mechanisms, and extensive applications, while underscoring the transformative impact it has across various sectors, including pharmaceuticals, environmental science, and food safety.
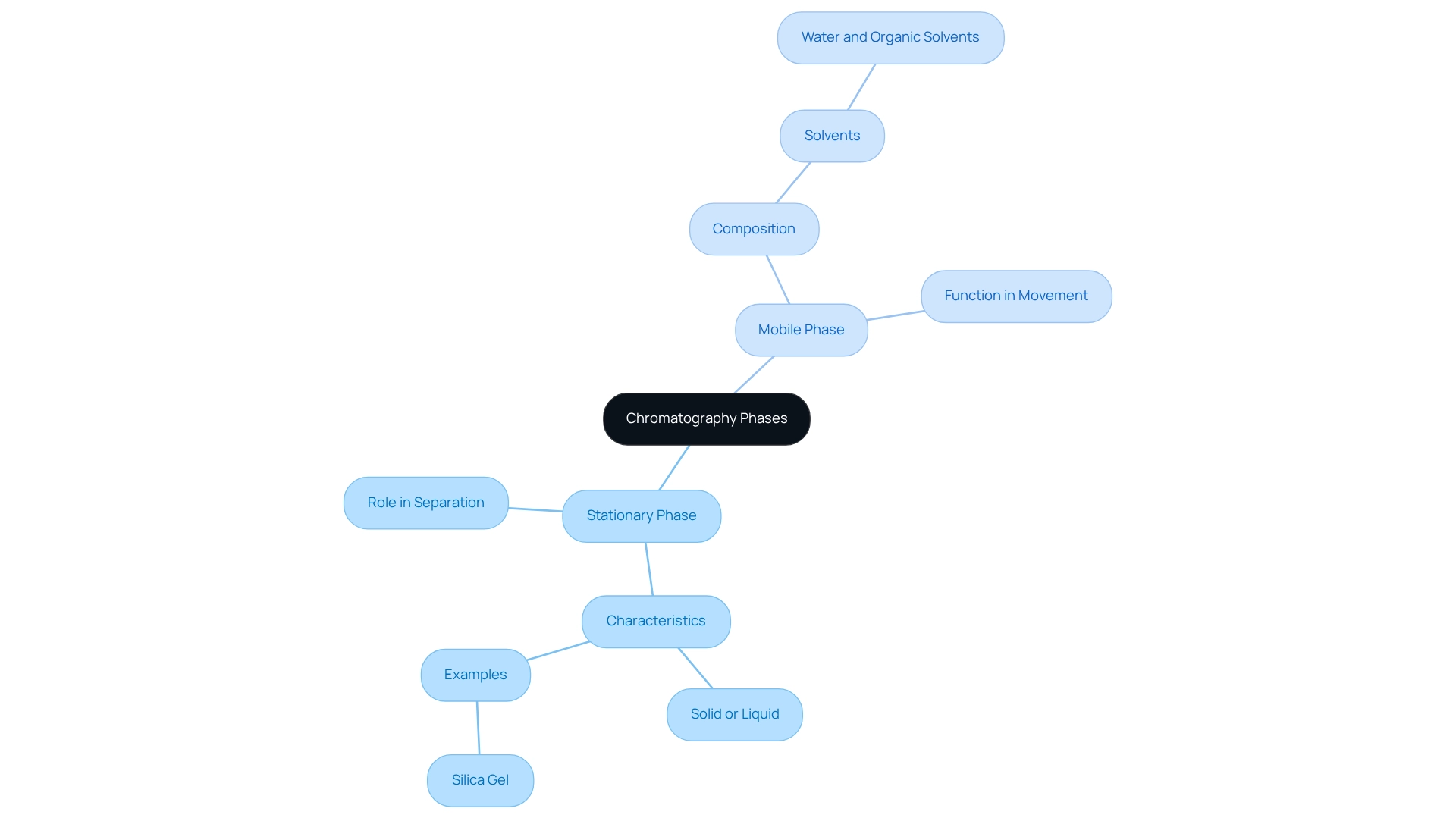
Define Stationary and Mobile Phases in Chromatography
In chromatography, the stationary phase mobile phase comprises the fixed material within the column or on a plate, which can be either a solid or a liquid coated onto a solid support. Conversely, the mobile medium, an integral part of the stationary phase mobile phase, refers to the solvent or gas that traverses the stationary medium, facilitating the movement of the mixture's components. The interaction between these two phases is crucial for isolating substances based on their differing affinities.
For instance, in liquid separation techniques, silica gel typically serves as the stationary medium, while the moving component may consist of a combination of water and organic solvents. This interaction is vital for achieving successful outcomes, as evidenced by case studies, including one titled 'Factors Influencing Chromatographic Processes,' which underscores that the choice of stationary phase mobile phase components significantly impacts efficiency. Recent advancements in chromatography focus on enhancing precision in analysis and sustainability, reflecting the evolving nature of these critical elements in analytical methods.
Experts emphasize that understanding the interactions between the stationary phase mobile phase and other moving components is essential for optimizing chromatographic separations. Furthermore, nonpolar solvents have proven to be more effective for the extraction and differentiation of nonpolar compounds, highlighting the practical implications of solvent selection in real-world applications.
Explain Chromatography Principles and Separation Mechanisms
Chromatography is fundamentally grounded in the principle of differential partitioning, where compounds are separated between the stationary phase and mobile phase. The specific separation mechanisms employed can vary significantly depending on the chromatography technique utilized.
- Adsorption Chromatography: This approach involves the adherence of mixture components to the stationary phase. The degree of adsorption directly influences the speed at which each component traverses the column, making it essential for achieving effective distinctions.
- Partition Chromatography: This technique relies on the separation of compounds between the stationary phase and mobile phase, a common practice in liquid chromatography. The effectiveness of this division is frequently enhanced by optimizing the interaction between the layers.
- Size Exclusion Chromatography: This method distinguishes molecules based on size, allowing smaller molecules to elute through the stationary phase while larger molecules are retained. This technique is particularly beneficial for purifying proteins and polymers.
Recent advancements in chromatography have underscored the effectiveness of various mechanisms for dividing components. For instance, gradient distinctions using advanced HPLC systems, such as those provided by JM Science Inc., illustrate operational parameters and detection techniques that yield accurate and reliable distinctions. This is crucial for analyzing intricate samples. JM Science offers a variety of high-quality HPLC columns and accessories, including Shoes and Capcell Pak columns, designed to enhance efficiency in drug-related applications.
This is particularly pertinent given that the minimum detection threshold established by existing regulations is 10, underscoring the necessity for efficient extraction methods in pharmaceutical applications.
Expert insights emphasize that understanding the stationary phase and mobile phase, along with these separation mechanisms quantitatively, is essential for reliable molecular identification in analytical techniques. As Mark R. Schure articulates, 'This coupling and its refinement towards achieving dependable molecular identification must be comprehended quantitatively in the context of separation techniques through the extension of SOT.' This statement highlights the significance of a quantitative method in separation science, which is vital for drug research.
Current trends reveal that certain combinations of reversed-phase liquid separation systems yield exceptionally high practical peak capacities due to their high column efficiencies. This is especially relevant in drug research, where the ability to effectively separate compounds can greatly influence drug development and analysis. Moreover, helium is frequently utilized as a carrier gas in gas separation techniques for examining luteolin derivatives, providing a practical illustration of separation applications in the drug industry.
In summary, a comprehensive understanding of separation principles and mechanisms is crucial for selecting the appropriate technique for specific applications, particularly within the pharmaceutical industry.
Discuss Applications of Chromatography in Scientific Research
Chromatography is a pivotal technique utilized across multiple scientific disciplines, including:
- Pharmaceutical Research: This field relies heavily on chromatography for the purification and analysis of drugs, ensuring their safety and efficacy. High-Performance Liquid Chromatography (HPLC) is especially typical for analyzing active medicinal components, with recent studies showing recoveries for new impurities ranging from 80.43% to 101.96%. This range is crucial as it directly affects the safety and effectiveness of medications, ensuring that products meet stringent regulatory standards. JM Science Inc. provides an extensive selection of high-quality HPLC columns, such as Shodex, CapcellPak, and Reprosil, which are essential for attaining optimal outcomes in drug analysis. The versatility of combined chromatographic techniques, as highlighted in the review titled "Utility of Combined Techniques in Pharmaceutical Analysis," underscores their effectiveness in quality control and stability assessments of pharmaceutical products.
- Environmental Monitoring: Chromatography plays a crucial role in detecting pollutants in water and soil samples, facilitating the assessment of environmental health. Recent advancements in gas separation techniques, often paired with mass spectrometry, have proven effective in identifying new bioactive compounds in plants, showcasing the method's adaptability in environmental applications. This combination is particularly valuable for monitoring specific pollutants and understanding their impact on ecosystems.
- Food and Beverage Industry: In this sector, separation techniques are utilized to analyze food components, ensuring quality and safety by detecting contaminants and verifying ingredient authenticity. This application is vital for maintaining consumer trust and regulatory compliance.
- Clinical Diagnostics: Chromatography is indispensable in medical laboratories for analyzing biological samples, aiding in disease diagnosis and monitoring. The incorporation of separation techniques in clinical environments enhances the accuracy of diagnostic processes, contributing to better patient outcomes.
These applications collectively illustrate the critical role chromatography plays in advancing scientific knowledge and ensuring public safety. Furthermore, the anticipated increase in scientific papers utilizing techniques such as TLC-MS and HPTLC-ESI-MS/MS indicates emerging trends and advancements in the field, promising to expand its utility in both pharmaceutical research and environmental monitoring.
Conclusion
The exploration of chromatography underscores its fundamental role in scientific research, illuminating the intricate relationship between stationary and mobile phases that are essential for effective separation and analysis. Understanding these phases is crucial, as they directly influence the efficiency and accuracy of chromatographic techniques. The principles of differential partitioning and various separation mechanisms—including adsorption, partition, and size exclusion chromatography—highlight the versatility of this method across diverse applications.
In the realm of pharmaceutical research, chromatography is pivotal in ensuring the safety and efficacy of drugs. Its applications extend to environmental monitoring, food safety, and clinical diagnostics. The ability to analyze complex mixtures with precision is vital not only for regulatory compliance but also for advancing scientific knowledge. As advancements in chromatography continue to evolve, the integration of modern techniques promises to enhance its utility further, reinforcing its indispensable nature across various sectors.
Ultimately, a comprehensive understanding of chromatography's principles and applications empowers researchers to harness its full potential, driving innovation and ensuring public safety. As the scientific community continues to explore and refine chromatographic methods, the transformative impact of this technique will undoubtedly persist, shaping the future of research across multiple disciplines.




