Overview
The equivalence point of titration is a critical moment defined as the juncture when the volume of titrant added is chemically equal to the quantity of analyte in the mixture, effectively marking the completion of the reaction. This point is not merely a technical detail; it is fundamental to achieving accurate measurements across various applications.
In particular, the pharmaceutical industry relies heavily on precise determination of active ingredient concentrations, which is essential for quality control and regulatory compliance.
The importance of understanding and identifying the equivalence point cannot be overstated—it is vital for ensuring the reliability of analytical results, thereby safeguarding product quality and efficacy.
Introduction
Understanding the intricacies of titration is essential for anyone involved in analytical chemistry. This technique plays a crucial role in determining the concentration of unknown substances. The equivalence point, a pivotal moment in this process, signifies the precise balance between titrant and analyte. Such accuracy is vital across various applications, ranging from pharmaceuticals to environmental testing. Yet, how does one effectively identify this critical juncture? What implications does it hold for the quality and safety of products? Recognizing these factors is paramount for ensuring reliable results in analytical endeavors.
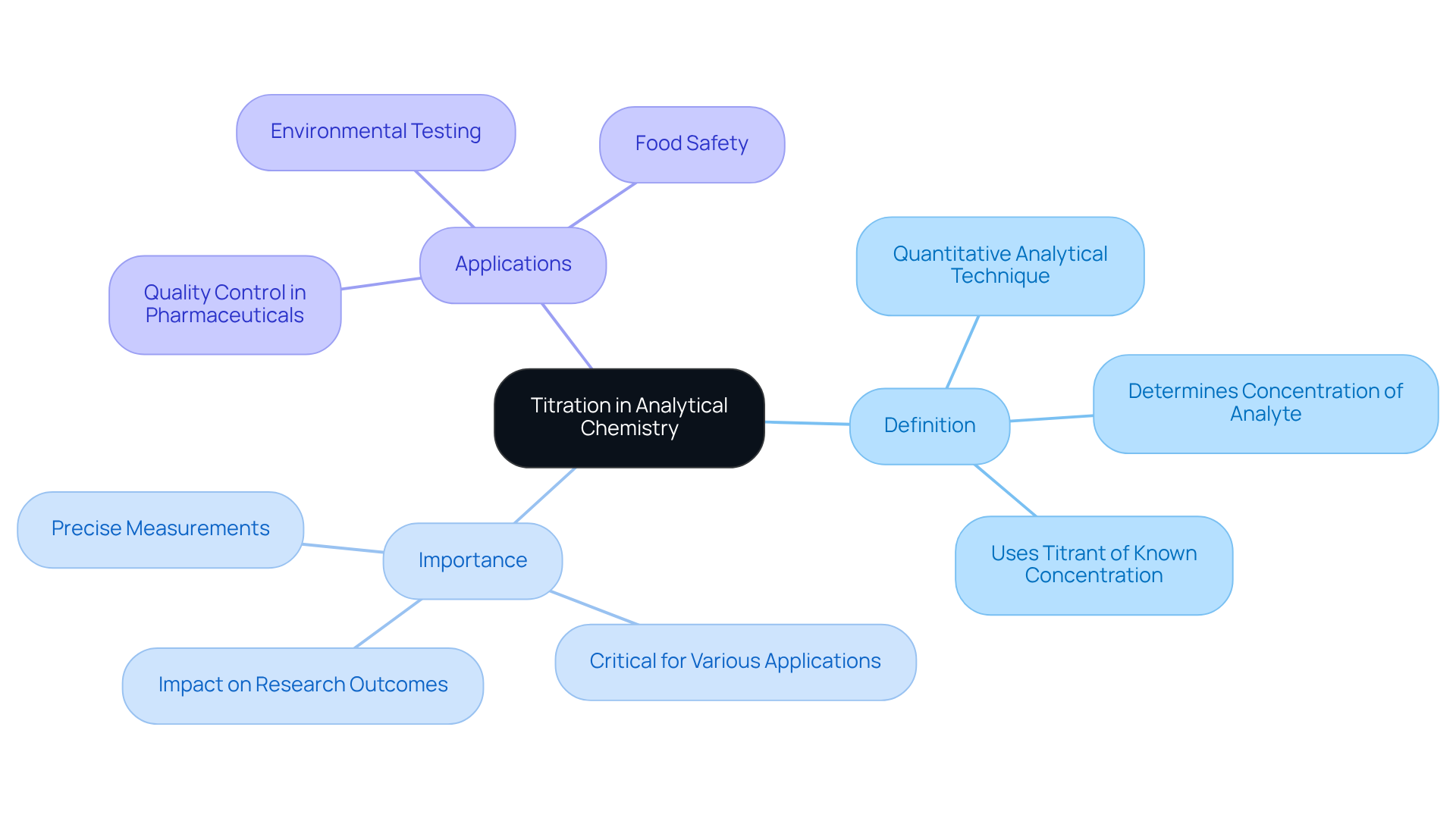
Define Titration and Its Importance in Analytical Chemistry
Titration stands as a pivotal quantitative analytical technique utilized to determine the concentration of an unknown substance, known as the analyte, by systematically adding a liquid of known concentration, referred to as the titrant, until the reaction reaches completion. This method is indispensable in analytical chemistry, offering precise measurements of chemical concentrations that are critical for a myriad of applications, including:
- Quality control in pharmaceuticals
- Environmental testing
- Food safety
JM Science Inc. excels in providing a diverse array of high-quality titrators, HPLC products, and accessories that significantly enhance the precision and effectiveness of measurement processes. The accuracy of volumetric analysis results can profoundly impact research outcomes and product formulations, underscoring the necessity of mastering this vital skill for laboratory professionals.
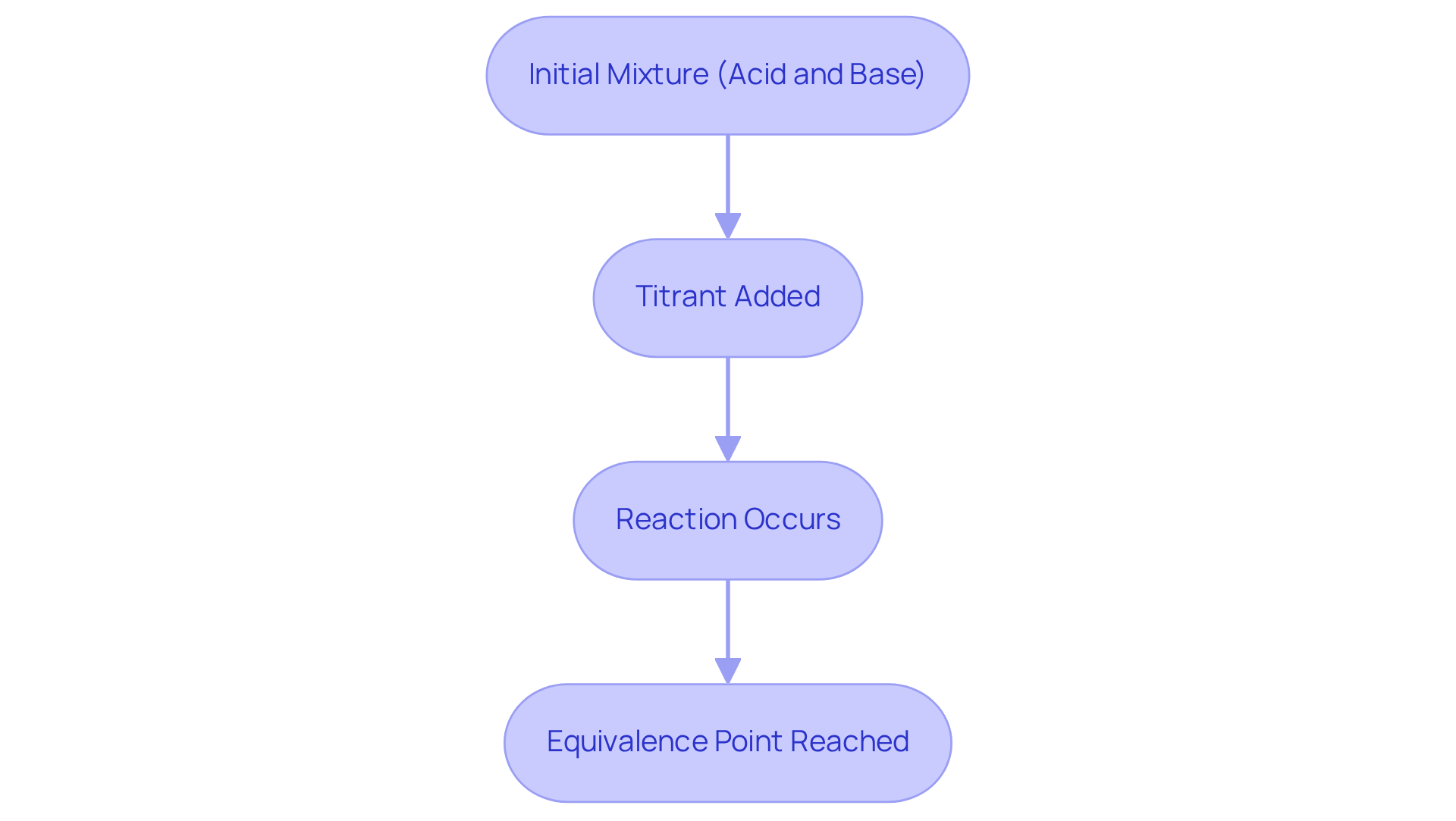
Explain the Equivalence Point and Its Role in Titration
The stage in a chemical analysis is defined as the equivalence point of titration, marked by the moment when the volume of titrant introduced is chemically equal to the quantity of analyte present in the mixture. At this pivotal stage, the interaction between the titrant and analyte concludes, resulting in a mixture that contains solely the products of the reaction.
For instance, during an acid-base neutralization, the moment of balance occurs when the amount of acid precisely matches the amount of base, yielding a neutral solution.
Identifying the equivalence point of titration is crucial for achieving precise results in the procedure, as it facilitates the calculation of the analyte's concentration based on the volume of reagent applied.
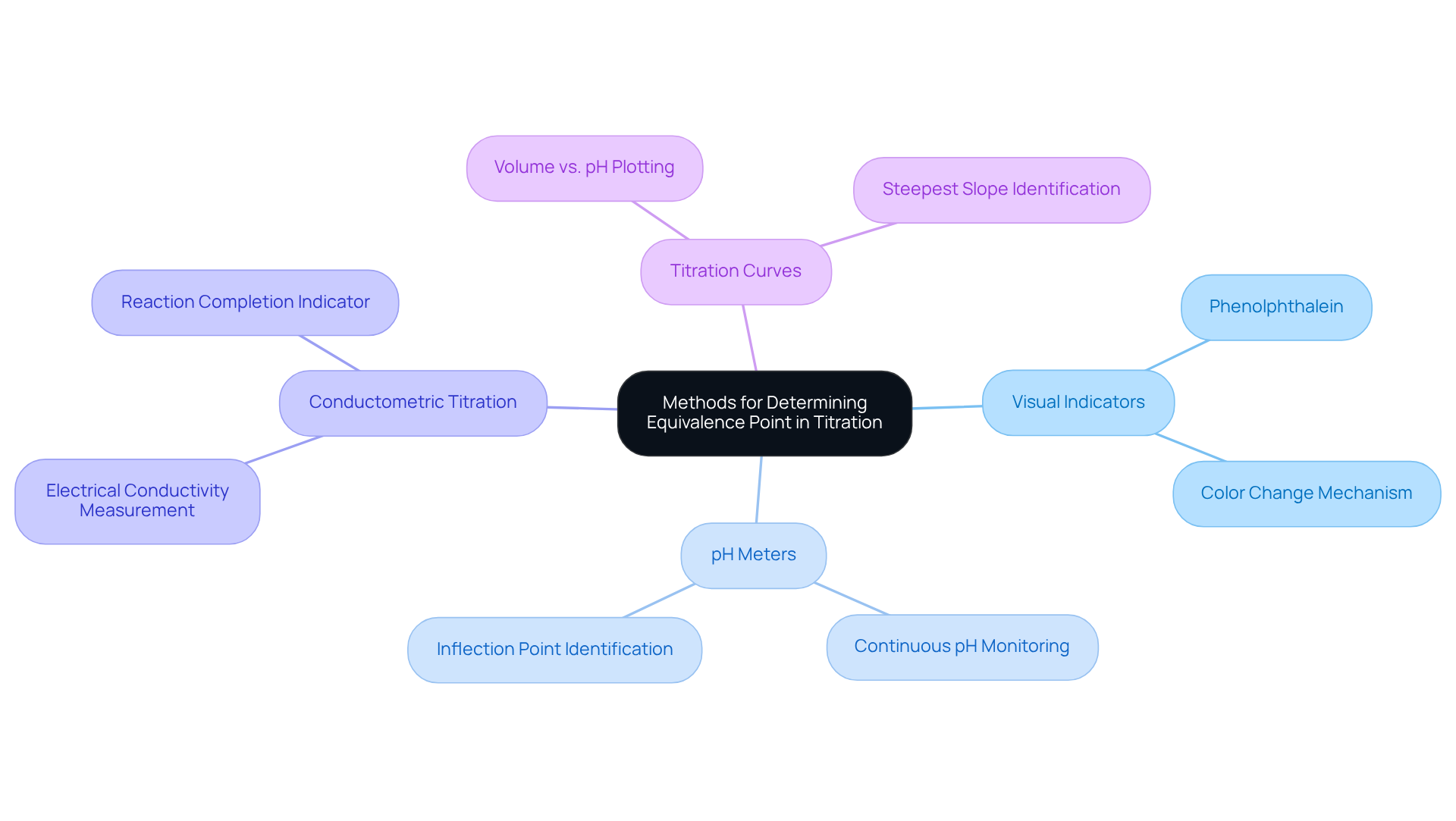
Describe Methods for Determining the Equivalence Point in Titration
Determining the equivalence point of titration is crucial for accurate results, and several methods can be employed to achieve this.
-
Visual Indicators are commonly utilized in acid-base titrations. These indicators change color at or near the equivalence point of titration, providing a visual cue. For example, phenolphthalein transitions from colorless to pink as the pH shifts from acidic to slightly basic, marking the endpoint effectively.
-
pH Meters offer a more precise technique, allowing for the continuous monitoring of pH as the titrant is introduced. The neutralization stage becomes apparent at the inflection point of the pH curve, which indicates the equivalence point of titration, where a sudden change in pH occurs.
-
Conductometric Titration measures the electrical conductivity of the solution. The equivalence point of titration is identified by a notable change in conductivity, which corresponds with the completion of the reaction, ensuring accuracy in the measurement.
-
Lastly, Titration Curves can be generated by plotting the volume of titrant added against the pH of the solution. The equivalence point of titration is recognized at the steepest slope of the curve, indicating a rapid change in pH. This visual representation reinforces the importance of understanding the titration process.
Each of these methods plays a vital role in achieving precise results in titration, underscoring the significance of selecting the appropriate technique for accurate analysis.
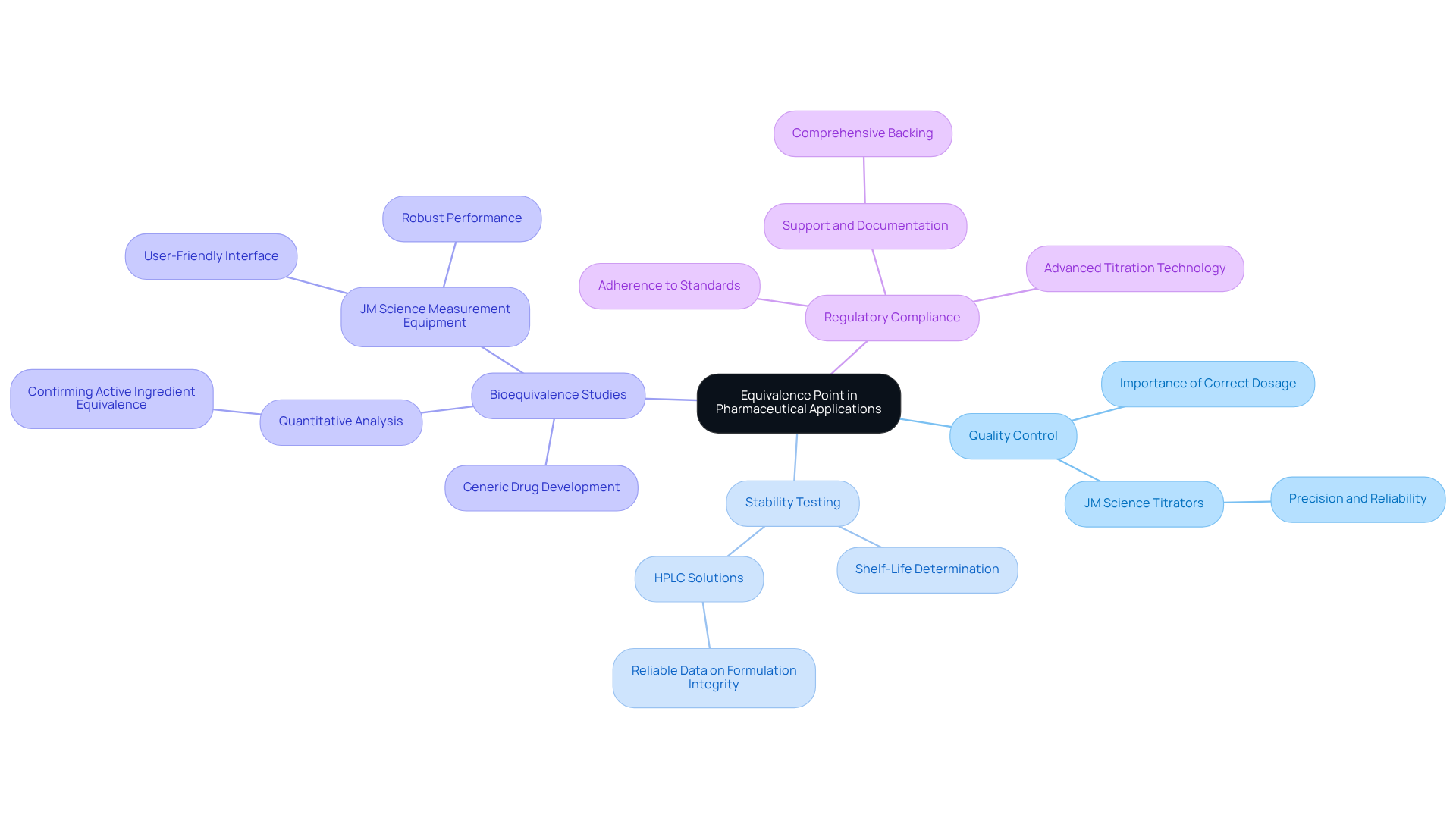
Discuss the Implications of the Equivalence Point in Pharmaceutical Applications
In the pharmaceutical sector, the matching stage is crucial for ensuring the precision and safety of medication formulations. Titration, for example, is employed to determine the concentration of active pharmaceutical ingredients (APIs) in formulations, ensuring compliance with regulatory standards. The accurate determination of the equivalence point is essential for several key areas:
-
Quality Control: Ensuring that drug products contain the correct dosage of active ingredients is vital for patient safety and efficacy. JM Science Inc. offers premium titrators designed for precision and reliability, significantly enhancing quality control measures within pharmaceutical laboratories.
-
Stability Testing: Understanding the equivalence point of titration is fundamental in assessing the stability of formulations over time, which is critical for determining shelf-life. The high-performance liquid chromatography (HPLC) solutions provided by JM Science are equipped with advanced features that deliver reliable data regarding formulation integrity during stability testing.
-
Bioequivalence Studies: In the realm of generic drug development, demonstrating that a generic product is bioequivalent to its brand-name counterpart often requires quantitative analysis to confirm that the active ingredients are present in equivalent amounts. JM Science's innovative measurement equipment supports these essential studies, ensuring accurate results through its user-friendly interface and robust performance.
-
Regulatory Compliance: Precise measurement outcomes are essential for adherence to regulatory standards, ensuring that pharmaceutical products are safe and effective for consumer use. With JM Science's advanced titration technology, pharmaceutical companies can confidently meet these stringent regulatory requirements, backed by comprehensive support and documentation.
Conclusion
The exploration of the equivalence point in titration underscores its fundamental role in achieving accurate chemical analysis. Understanding this critical juncture, where the titrant and analyte react in exact proportions, is essential for obtaining reliable results across various applications, particularly in analytical chemistry and pharmaceuticals.
Throughout this discussion, key methods for determining the equivalence point have been highlighted, including:
- Visual indicators
- pH meters
- Conductometric titration
- Titration curves
Each technique presents unique advantages, ensuring that the precise moment of reaction completion is accurately identified. The implications of this knowledge extend into vital areas such as quality control, stability testing, and regulatory compliance within the pharmaceutical industry, where the safety and efficacy of drug formulations depend on accurate concentration measurements.
Recognizing the significance of the equivalence point not only enhances laboratory practices but also emphasizes the broader impact of precise analytical techniques on public health and safety. Embracing these methodologies, alongside the technology provided by industry leaders like JM Science, empowers professionals to uphold rigorous standards in their work, ensuring that the products reaching consumers are both safe and effective.




