Overview
The peroxidation index (PI) of oils serves as a crucial measure of oxidation levels, providing essential insights into the freshness and safety of fats. By quantifying peroxide concentrations in milliequivalents of active oxygen per kilogram of oil, the PI plays a pivotal role in food quality assessment. Monitoring the PI is vital to prevent rancidity and mitigate harmful health effects, as elevated peroxide levels correlate with food spoilage and potential toxicity. This reality underscores the necessity for rigorous quality control within the food and cosmetic industries. Emphasizing the importance of the PI not only highlights its role in ensuring product safety but also advocates for the implementation of stringent monitoring practices to uphold industry standards.
Introduction
Understanding the peroxidation index of oils is crucial for maintaining quality and safety across various industries, particularly in food and cosmetics. This measurement acts as a key indicator of oxidation levels, signaling both the freshness and degradation of oils. Such changes directly impact taste, nutritional value, and ultimately, consumer health. Alarmingly, statistics reveal that a significant percentage of products exceed safe peroxide levels. Consequently, industries must ask: how can they effectively monitor and control the peroxidation index to ensure product integrity and protect consumers?
Define the Peroxidation Index and Its Importance in Oil Analysis
The peroxide measurement (PI) serves as a critical indicator of oxidation levels in fats, quantifying the concentration of peroxides present. Expressed in milliequivalents of active oxygen per kilogram of oil (meq O2/kg), a low PI signifies fresh oil with minimal oxidation, whereas a high PI indicates significant degradation, which can lead to rancidity and the formation of potentially harmful compounds. Monitoring this oxidation measurement is essential for ensuring the quality and safety of fats, particularly in the food and cosmetic industries, where oxidized fats can negatively impact taste, nutritional value, and consumer health.
Recent studies reveal that 83% of tested products exceeded the recommended peroxide value levels, highlighting the urgent need for stringent control measures. Naohiro Gotoh emphasizes that elevated peroxide levels are linked to adverse health effects, including food poisoning, underscoring the necessity for continuous monitoring. Current methodologies for assessing the peroxidation index of oils utilize advanced analytical techniques such as gas chromatography and spectrophotometry, which provide accurate evaluations that comply with international safety standards.
The practical applications of are particularly evident in the food sector, where producers are increasingly adopting rigorous standards to maintain product integrity and bolster consumer confidence. Ideally, freshly refined fats should exhibit a peroxide value around 10 mEq/kg, while rancid fats exceed 30 mEq/kg, establishing clear benchmarks for acceptable quality standards.
Explain the Chemistry of Oil Peroxidation and Its Implications
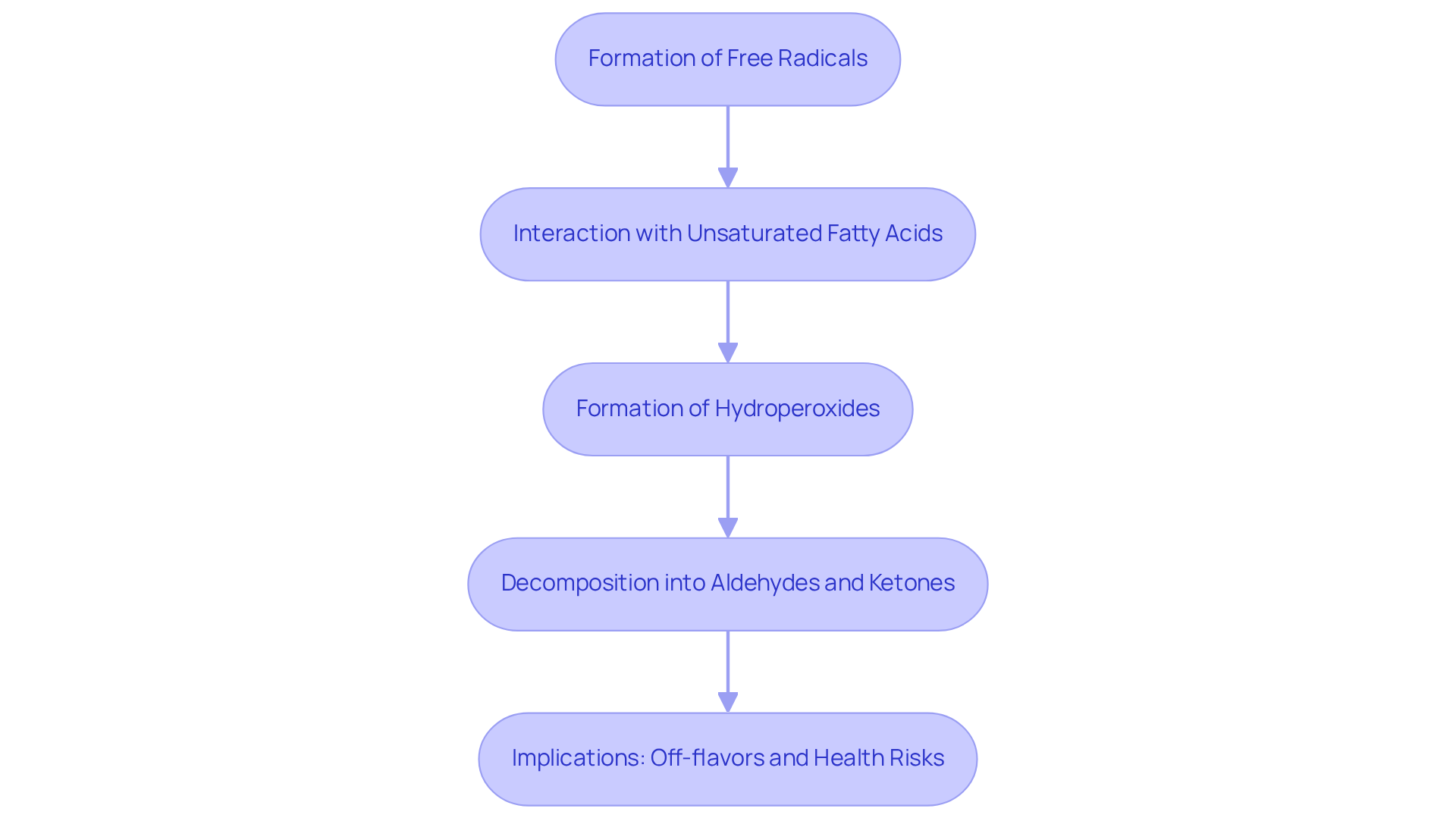
The peroxidation index of oils reflects a complex chemical reaction involving the interaction of unsaturated fatty acids with oxygen. This intricate process initiates with the formation of free radicals, which possess the ability to extract electrons from lipid molecules, subsequently leading to the creation of hydroperoxides. These hydroperoxides can decompose into various secondary oxidation products, such as aldehydes and ketones, which are notorious for contributing to off-flavors and rancidity.
The implications of the peroxidation index of oils are profound; it not only influences the sensory characteristics of oils but also generates harmful compounds that pose significant health risks. Understanding these chemical reactions is crucial for developing effective strategies to prevent oxidation and maintain oil quality.
Detail the Equipment and Procedures for Measuring the Peroxidation Index
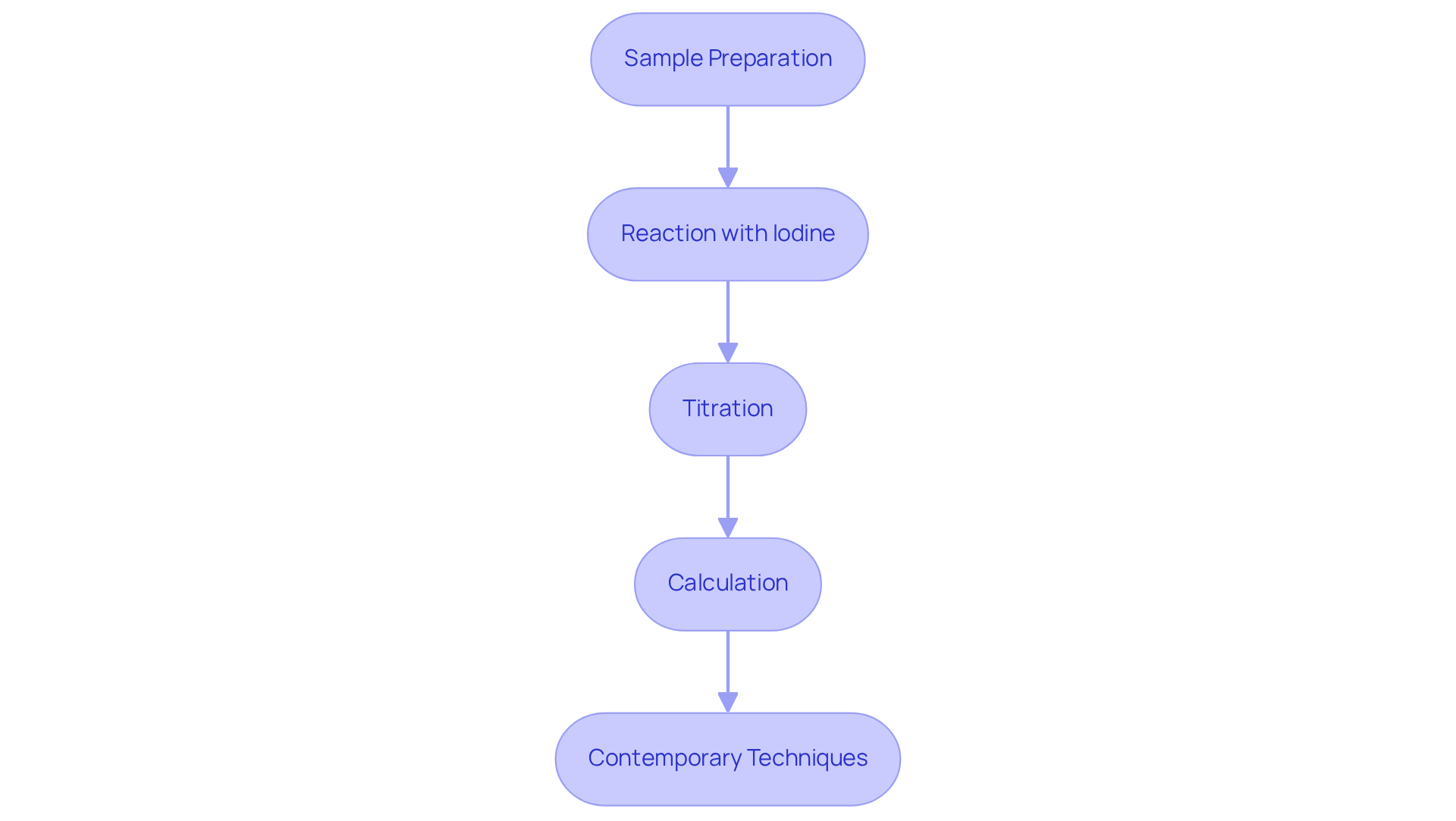
To assess the peroxidation index of oils, several techniques can be employed, with iodometric titration being the most widely used. This procedure encompasses several critical steps:
- Sample Preparation: Begin by taking a representative oil sample and diluting it with , typically a mixture of acetic acid and chloroform.
- Reaction with Iodine: Introduce a potassium iodide solution to the oil sample. The peroxides present will oxidize the iodide ions to iodine, initiating the reaction.
- Titration: Proceed to titrate the liberated iodine with a sodium thiosulfate solution. The endpoint is indicated by a color change, and the volume of sodium thiosulfate used directly correlates to the peroxidation index of oils.
- Calculation: Finally, determine the oxidation value using the formula: PI (meq O2/kg) = (Volume of Na2S2O3 used × Normality of Na2S2O3 × 1000) / Weight of the oil sample (kg).
In addition to iodometric titration, contemporary techniques such as spectrophotometry and electronic sensors can provide rapid and precise measurements of the peroxidation index of oils. These advancements significantly enhance laboratory efficiency, ensuring accurate assessments of peroxide levels in oils.
Interpret Results and Understand the Role of the Peroxidation Index in Quality Control
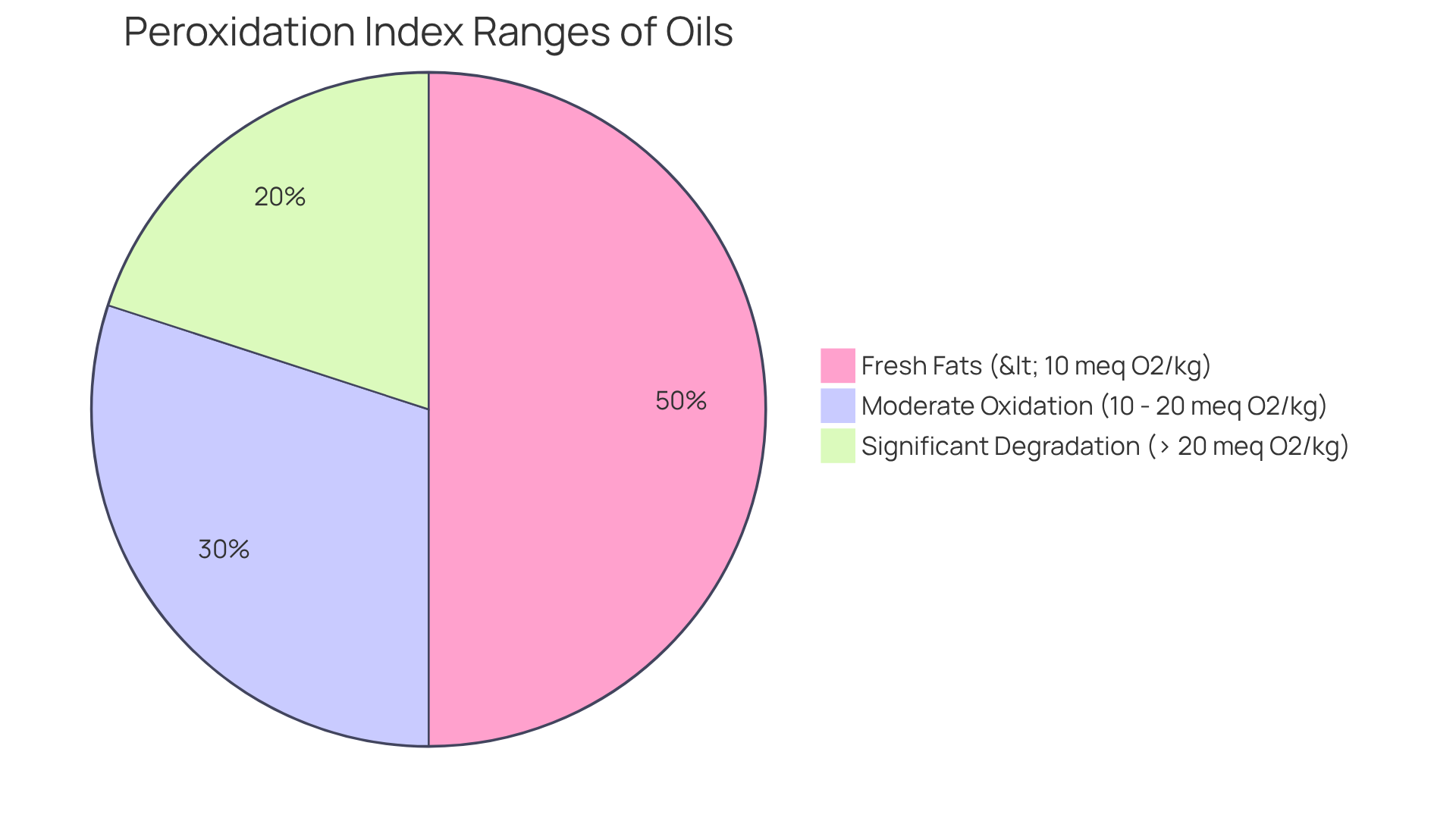
Interpreting the results of the peroxidation index of oils necessitates a clear understanding of the acceptable ranges for various types of fats. Fresh fats typically exhibit a PI of less than 10 meq O2/kg, indicating minimal oxidation. Values ranging from 10 to 20 meq O2/kg suggest moderate oxidation, while those exceeding 20 meq O2/kg signal . Notably, research indicates that a fat is considered rancid when the PI reaches approximately 10 meq/kg. This underscores the critical need for vigilant oversight to avert health hazards linked to oxidized fats.
Regular monitoring of the peroxidation index of oils is essential in control measures to ensure product safety and compliance with industry standards. Establishing a baseline for acceptable peroxidation index of oils levels empowers laboratories to take corrective actions when values exceed these thresholds, thereby preserving product integrity and safeguarding consumer health.
Furthermore, it is crucial to note that the peroxide values for fresh vegetable fats range from 0.60 to 5.33 meq/kg, emphasizing the variability in oxidation products among different types. Additionally, heating vegetable oils can significantly elevate alkenal concentrations, which is pertinent to discussions surrounding oil quality and oxidation.
Conclusion
Understanding the peroxidation index of oils is essential for maintaining quality and safety across various industries, particularly in food and cosmetics. This metric serves as a crucial barometer for oxidation levels, ensuring that oils remain fresh and safe for consumption. By effectively monitoring the peroxidation index, producers can prevent rancidity and the formation of harmful compounds, ultimately safeguarding consumer health.
Throughout this article, we have explored key aspects of the peroxidation index, including its definition, the chemistry behind oil peroxidation, and the methodologies for measuring this critical parameter. The significance of adhering to established peroxide value benchmarks has been emphasized, alongside the necessity for advanced analytical techniques to provide accurate assessments. The implications of elevated peroxide levels on oil quality and health risks further underscore the importance of rigorous quality control measures.
In conclusion, the peroxidation index is not merely a technical measurement; it is a fundamental aspect of oil quality assurance. As industries continue to prioritize product integrity and consumer safety, a commitment to regular monitoring and adherence to quality standards is essential. By embracing best practices in peroxidation index analysis, stakeholders can ensure that oils meet the highest safety and quality benchmarks, thereby fostering consumer trust and promoting public health.




