Overview
Titration stands as a fundamental quantitative analytical technique in chemistry, essential for determining the concentration of a solute in a solution. This is achieved through the systematic addition of a known concentration solution until a reaction endpoint is reached. The significance of titration resonates across various scientific fields, particularly in pharmaceuticals. Here, precise measurements are paramount, ensuring drug safety and compliance with regulatory standards. This highlights the critical role of titration in accurate quantitative analysis, reinforcing the necessity of high-quality scientific instruments in laboratory settings.
Introduction
Titration serves as a cornerstone in the realm of chemistry, representing a precise method for determining the concentration of solutes in solutions. This analytical technique not only addresses fundamental scientific inquiries but also plays a pivotal role in critical industries, such as pharmaceuticals and environmental science. As technology continues to evolve, it prompts an essential question: how do modern advancements in titration methods enhance accuracy and efficiency? Furthermore, what challenges arise in ensuring safety and reliability within laboratory practices? These inquiries underscore the importance of high-quality scientific instruments in laboratory settings, inviting further exploration into the advancements shaping this vital field.
Define Titration: Core Concept in Chemistry
The concept of titration meaning in chemistry refers to a fundamental quantitative analytical technique utilized to ascertain the concentration of a solute within a solution. This method, which exemplifies titration meaning in chemistry, involves the systematic addition of a titrant—a solution with a known concentration—to a sample solution until the chemical reaction reaches completion, typically indicated by a color change or a specific measurement. The equivalence point marks this critical juncture in the reaction, underscoring the importance of precision in scientific analysis. The concept of titration meaning in chemistry plays a vital role across various scientific disciplines, including biology and environmental science, ensuring accurate measurements and analyses.
In practice, volumetric analysis experiments are meticulously designed to indirectly assess the concentration of an analyte by gradually introducing a titrant. For optimal accuracy, it is recommended that these experiments be repeated at least three times per analyte, reinforcing the reliability of the results. The versatility of this method is evident in its applications, such as titration meaning in chemistry, which includes acid-base tests where a strong acid interacts with a strong base to achieve a neutral pH at the conclusion. Other types encompass precipitation analyses, complex-formation tests, and oxidation-reduction (redox) procedures, each serving distinct analytical purposes.
In the pharmaceutical industry, the titration meaning in chemistry is essential for ensuring the quality and compliance of medications and therapies. Instruments such as the AQ-300 Coulometric Karl Fischer Titrator and the Hiranuma Aquacounter AQV-300 Volumetric Titrator from JM Science are specifically designed for these applications, adhering to the rigorous standards set by the Japanese Pharmacopoeia. These advanced instruments facilitate precise moisture content assessment in medications, which is crucial for product stability and effectiveness. The AQ-300 features advanced automation capabilities, while the AQV-300 offers high precision in volumetric measurements, making them ideal for pharmaceutical testing.
It is crucial to acknowledge that these experiments involve handling corrosive, toxic, and hazardous substances, which pose risks such as chemical burns and poisoning. Proper safety precautions—including the use of goggles, gloves, and lab gowns—are essential to safeguard against these dangers.
Recent advancements in measurement technology, particularly automated systems, have significantly enhanced efficiency and reproducibility, enabling high-throughput analyses with minimal operator intervention. This evolution underscores the significance of the titration meaning in chemistry within contemporary analytical chemistry, as it remains a fundamental method for precise and dependable quantitative analysis in laboratory environments.
Trace the History and Evolution of Titration Techniques
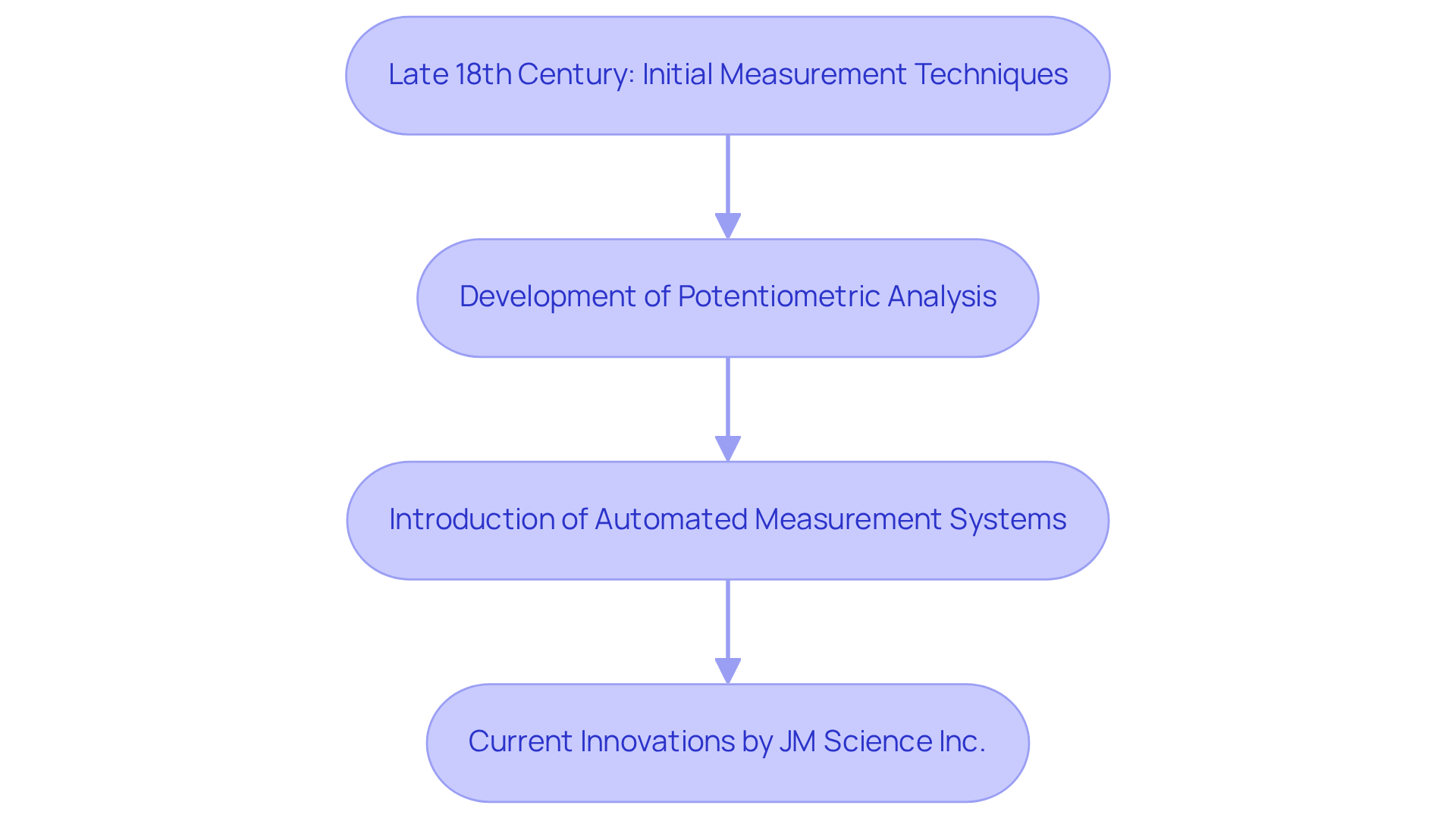
The origins of this technique can be traced back to the late 18th century, when chemists began developing methods for accurately measuring solution concentrations. Initial measurement techniques were rudimentary, relying on visual cues and basic assessments. Over the years, advancements in instrumentation and analytical methods have led to the emergence of sophisticated techniques such as potentiometric analysis and automated measurement systems.
JM Science Inc. has played a crucial role in this evolution, offering high-quality titrators, Karl Fischer reagents, and HPLC columns that significantly enhance measurement accuracy and efficiency in laboratory settings. For instance, potentiometric analysis allows for precise endpoint detection through electrode use, markedly improving measurement precision.
Moreover, automated dispensing systems, including those supplied by JM Science, have revolutionized laboratory practices by streamlining workflows and reducing human error. These innovations have made accurate measurement indispensable in modern laboratories, particularly in drug development and environmental assessments, where precision is paramount.
The evolution of titration techniques illustrates the titration meaning in chemistry and exemplifies a broader trend in scientific instrumentation, with continuous technological advancements from JM Science expanding applications and capabilities across diverse fields.
Explore Different Types of Titration Methods and Their Applications
The titration meaning in chemistry is exemplified by various approaches that represent vital analytical techniques, each meticulously designed for specific applications in chemistry and medicine. The primary types include:
-
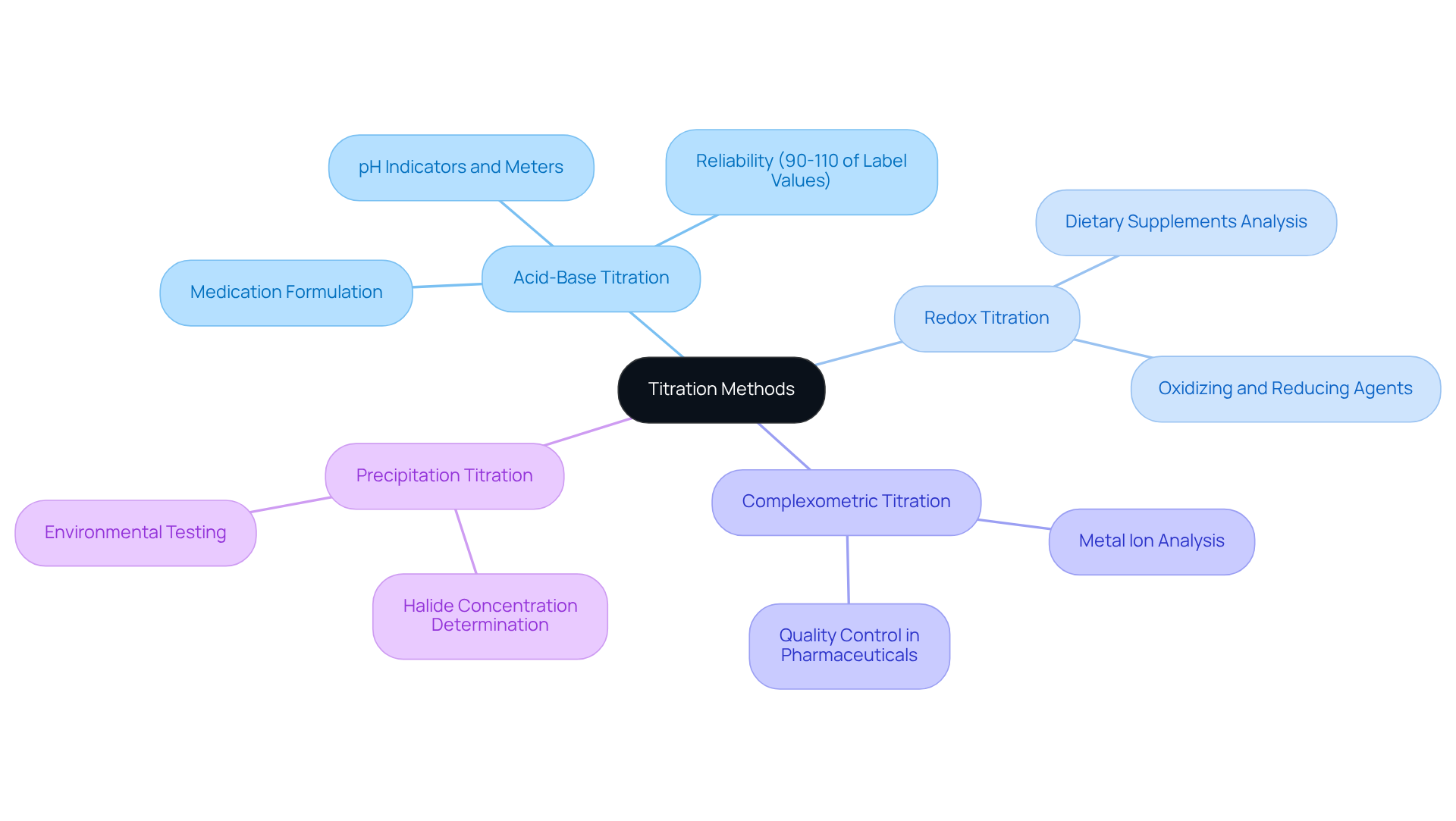
Acid-Base Titration: This method is instrumental in determining the concentration of acidic or basic solutions, utilizing pH indicators or meters to accurately identify the endpoint. It is widely employed in laboratory settings to ensure the precise formulation of medications, with results generally falling within 90-110% of label values according to USP standards. Notably, the titration techniques for bismuth subsalicylate in over-the-counter stomach relief products also yield results within this range, underscoring the reliability of acid-base titration in pharmaceutical applications.
-
Redox Titration: This technique, which involves oxidation-reduction reactions, is crucial for quantifying oxidizing or reducing agents. For example, it is commonly utilized in the analysis of dietary supplements, where the concentration of active ingredients must be measured with precision.
-
Complexometric Titration: Employing complexation reactions, this method is particularly effective in metal ion analysis. It is frequently applied in quality control processes within pharmaceutical manufacturing, ensuring that metal contaminants remain within acceptable limits.
-
Precipitation Titration: This method involves the formation of a precipitate and is particularly adept at determining halide concentrations. It finds extensive application in environmental testing and food safety assessments, where accurate quantification of halides is essential.
Each titration approach employs specific indicators and techniques, which helps illustrate titration meaning in chemistry as a versatile tool in analytical chemistry. Recent studies have demonstrated that the Mohr method consistently yields higher concentration values compared to the Volhard and Fajans methods, emphasizing the critical nature of method selection in achieving accurate results. This finding is especially significant in laboratory environments, where precision is paramount. Furthermore, the integration of contemporary technology, such as smartphone applications for tracking measurements, enhances accessibility and efficiency in laboratory settings, facilitating real-time data analysis and improving educational outcomes.
Understand the Importance of Titration in Pharmaceutical and Medical Laboratories
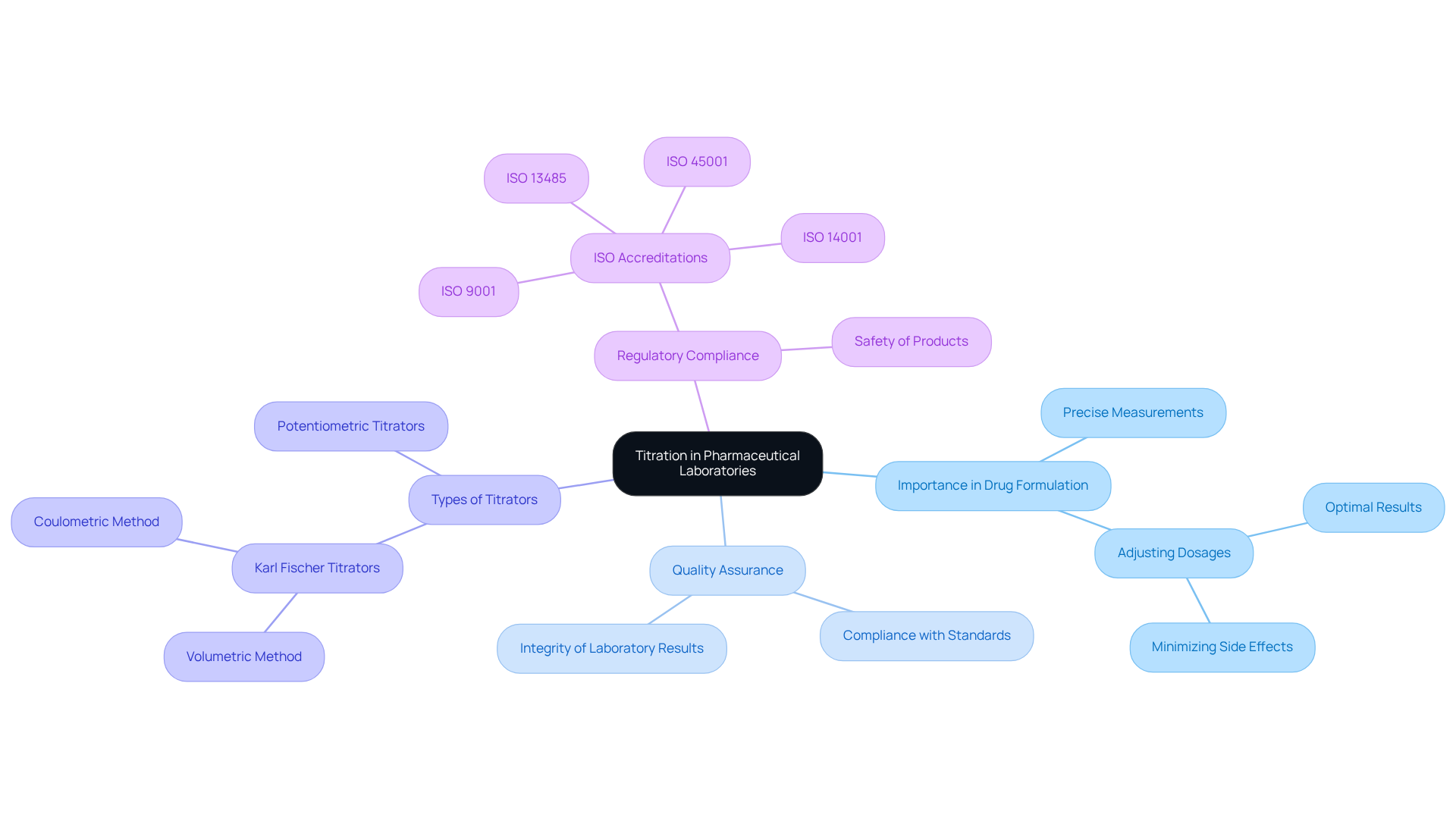
In pharmaceutical and medical laboratories, the titration meaning in chemistry is indispensable, as precise measurements are critical for drug formulation and quality assurance. JM Science Inc. provides a comprehensive selection of premium titrators, including Karl Fischer titrators and potentiometric titrators, essential for accurately determining the concentration of active ingredients in medications. These instruments not only ensure compliance with regulatory standards but also guarantee the safety of products for patient use.
Furthermore, precise measurement is crucial in the development of new drugs, where meticulous analysis of chemical compounds is vital for achieving successful outcomes. The reliability of titration methods, bolstered by JM Science's innovative solutions, significantly enhances the integrity of laboratory results, solidifying the titration meaning in chemistry as a cornerstone technique within the scientific community.
Conclusion
The essence of titration in chemistry lies in its ability to provide precise quantitative analysis of substance concentrations within solutions. This fundamental technique not only facilitates accurate measurements but also plays a crucial role across various scientific disciplines, including pharmaceuticals and environmental science. By systematically adding a titrant to a solution until a reaction reaches completion, titration serves as a cornerstone in ensuring the reliability of experimental results.
Throughout this article, key aspects of titration have been explored, from its historical evolution to the various methods employed in modern laboratories. The significance of acid-base, redox, complexometric, and precipitation titrations has been highlighted, showcasing their diverse applications in fields such as drug formulation, quality control, and environmental testing. Furthermore, advancements in technology and instrumentation, particularly from industry leaders like JM Science, have transformed titration practices, enhancing accuracy and efficiency in laboratory settings.
In reflection, the importance of titration extends beyond mere measurement; it underpins the integrity of scientific research and product safety. As the demand for precision in analytical chemistry continues to grow, embracing innovative titration techniques and technologies will be vital. Engaging with these advancements not only ensures compliance with regulatory standards but also enhances the overall quality of scientific inquiry and healthcare solutions.




