Overview
IR spectroscopy is a pivotal technique that measures the interaction between infrared radiation and matter, with a specific focus on the vibrational transitions of molecules. This method allows for the precise identification of molecular structures and functional groups. The technique is grounded in the principles of absorption, emission, and reflection of infrared light, resulting in a spectrum that acts as a unique fingerprint for analyzing a diverse array of compounds. Its applications span critical fields such as chemistry, biology, and materials science, underscoring its significance in laboratory settings. By understanding these interactions, scientists can unlock valuable insights into the composition and behavior of various substances.
Introduction
Spectroscopy stands at the forefront of scientific analysis, unraveling the complexities of molecular interactions through the lens of infrared radiation. This powerful technique not only measures the vibrational transitions of molecules but also provides a unique fingerprint of their structure. Such capabilities open doors to a myriad of applications across chemistry, biology, and materials science.
What exactly does IR spectroscopy measure? How do its principles translate into real-world applications? Understanding these nuances reveals the critical role this technique plays in everything from drug development to environmental monitoring. This invites exploration into its fascinating capabilities.
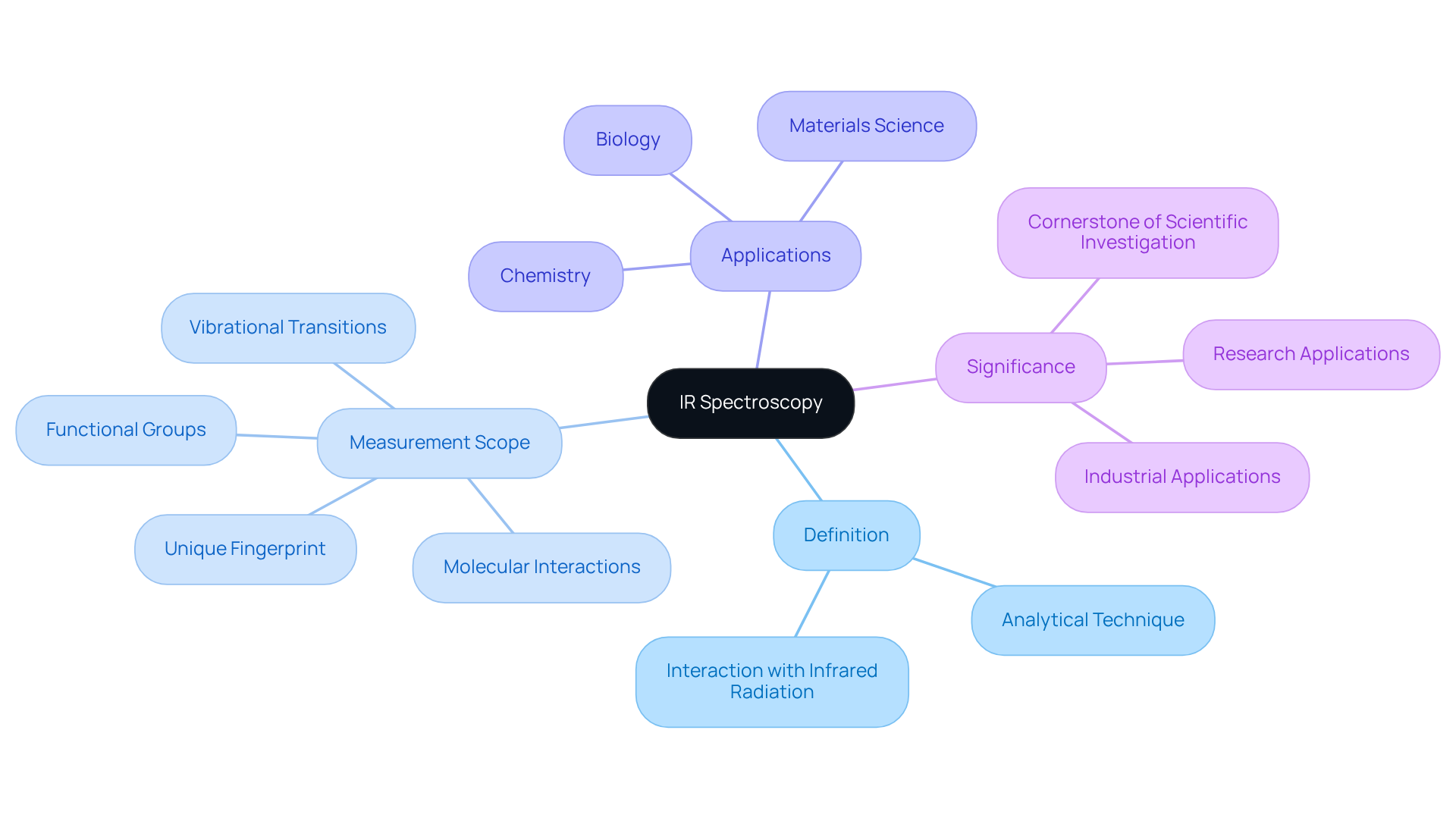
Define IR Spectroscopy and Its Measurement Scope
Spectroscopy is a powerful analytical technique that evaluates what does IR spectroscopy measure in terms of the interaction between infrared radiation and matter, with a primary focus on the vibrational transitions of molecules. By transmitting light through a sample, this method identifies specific wavelengths that are absorbed, emitted, or reflected. The resulting spectrum serves as a unique fingerprint of the molecular structure, facilitating the identification of functional groups and molecular interactions, which illustrates what does IR spectroscopy measure.
Widely utilized across various fields, including chemistry, biology, and materials science, this technique is essential for analyzing both organic and inorganic compounds. Its significance in research and industrial applications cannot be overstated, making it a cornerstone of modern scientific investigation.
Explore the Principles of IR Spectroscopy
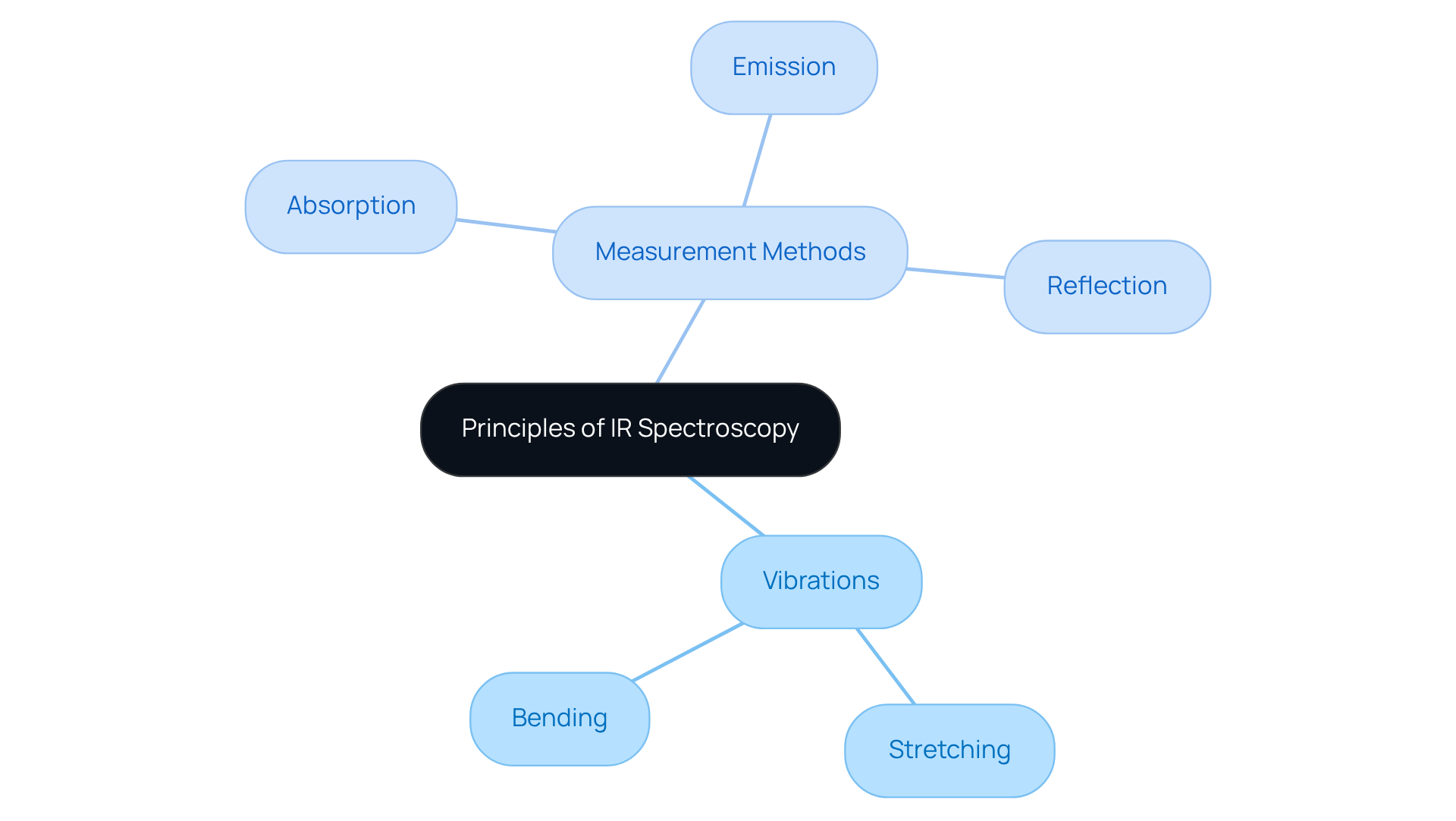
The fundamental principle of IR spectroscopy is based on the absorption of infrared light by substances, which induces specific bonds within them to vibrate. These vibrations can be categorized into stretching and bending modes. For a molecule to absorb IR radiation, it must possess a dipole moment; this means that the distribution of electrical charge within the molecule must change during vibration. The resulting spectrum displays peaks that correspond to the frequencies of absorbed light, which can be analyzed to determine the types of bonds and functional groups present in the sample. This interaction is typically measured in three ways:
- Absorption
- Emission
- Reflection
Each providing valuable insights into the molecular structure.
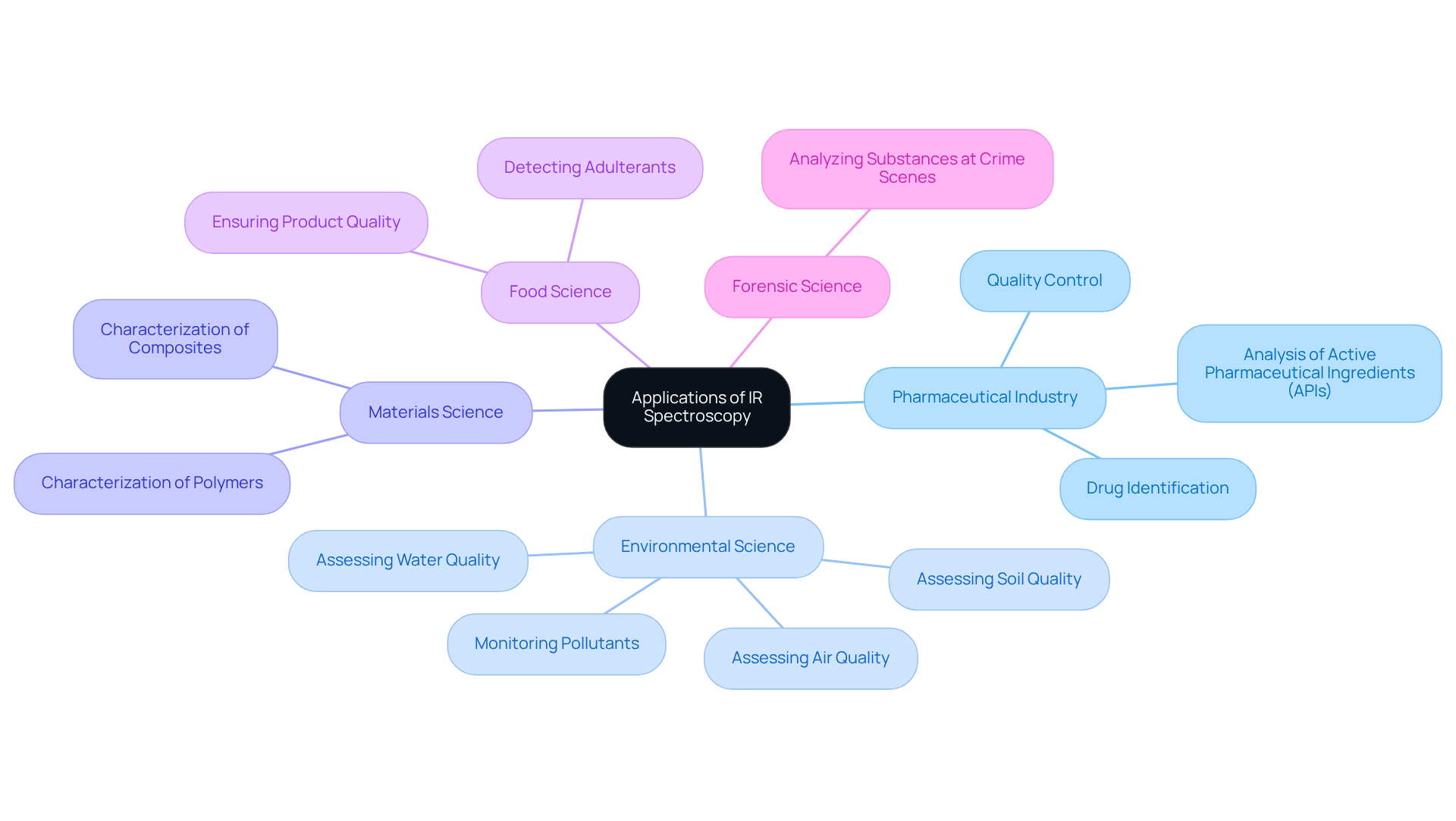
Examine Applications of IR Spectroscopy in Various Fields
Infrared (IR) analysis serves as a pivotal tool across a multitude of applications in diverse fields, especially when considering what does IR spectroscopy measure. In the pharmaceutical industry, it is instrumental for:
- Drug identification
- Quality control
- The analysis of active pharmaceutical ingredients (APIs)
Environmental scientists leverage infrared analysis to monitor pollutants and assess the quality of air, water, and soil. In materials science, this technique aids in the characterization of polymers and composites, while in food science, it plays a critical role in detecting adulterants and ensuring product quality. Furthermore, in forensic science, what does IR spectroscopy measure is essential for analyzing substances found at crime scenes. Its capacity to deliver rapid and reliable data underscores its invaluable role in both research and practical applications.
Conclusion
IR spectroscopy serves as a pivotal analytical technique, illuminating the complex interactions between infrared radiation and matter, with a primary focus on the vibrational transitions of molecules. By offering a distinct spectral fingerprint of molecular structures, it facilitates the identification of functional groups and molecular interactions, thereby cementing its status as a foundational tool across diverse scientific disciplines.
The article delved into the principles of IR spectroscopy, illustrating how molecules absorb infrared light to induce vibrations measurable through absorption, emission, and reflection. This methodology not only aids in identifying the types of bonds and functional groups present in a sample but also showcases the versatility of IR spectroscopy in various fields, including:
- Pharmaceuticals
- Environmental science
- Materials science
- Forensic analysis
Each application emphasizes the technique’s capacity to deliver rapid and reliable results, rendering it indispensable in both research and industry.
Ultimately, the importance of IR spectroscopy transcends mere measurement; it equips scientists and researchers with the tools to unravel the complexities of molecular interactions and enhance product quality across multiple sectors. By embracing this powerful technique, advancements in understanding chemical properties and improving processes can be achieved, underscoring the vital role of IR spectroscopy in contemporary scientific inquiry.




