Overview
Reversed High-Performance Liquid Chromatography (HPLC) stands as a pivotal analytical technique, adept at separating substances based on their hydrophobicity. By utilizing a non-polar stationary phase alongside a polar mobile phase, HPLC achieves effective separation of compounds. This article meticulously outlines the core principles of HPLC, tracing its historical evolution and showcasing its diverse applications across:
- Pharmaceutical analysis
- Environmental monitoring
- Clinical diagnostics
Such applications underscore HPLC's critical role in ensuring quality control and regulatory compliance within various scientific fields, establishing it as an indispensable tool for researchers and practitioners alike.
Introduction
Reversed High-Performance Liquid Chromatography (HPLC) stands as a cornerstone in analytical chemistry, fundamentally transforming the methods by which substances are separated and analyzed. This technique harnesses the unique interactions between non-polar and polar compounds, significantly enhancing the accuracy of pharmaceutical assessments. Moreover, it plays an essential role in environmental monitoring and clinical diagnostics, showcasing its versatility across various scientific domains.
However, as the demand for precision in scientific research intensifies, it raises a critical question: how can researchers effectively navigate the inherent challenges of reversed HPLC to unlock its full potential?
Define Reversed HPLC: Core Principles and Mechanisms
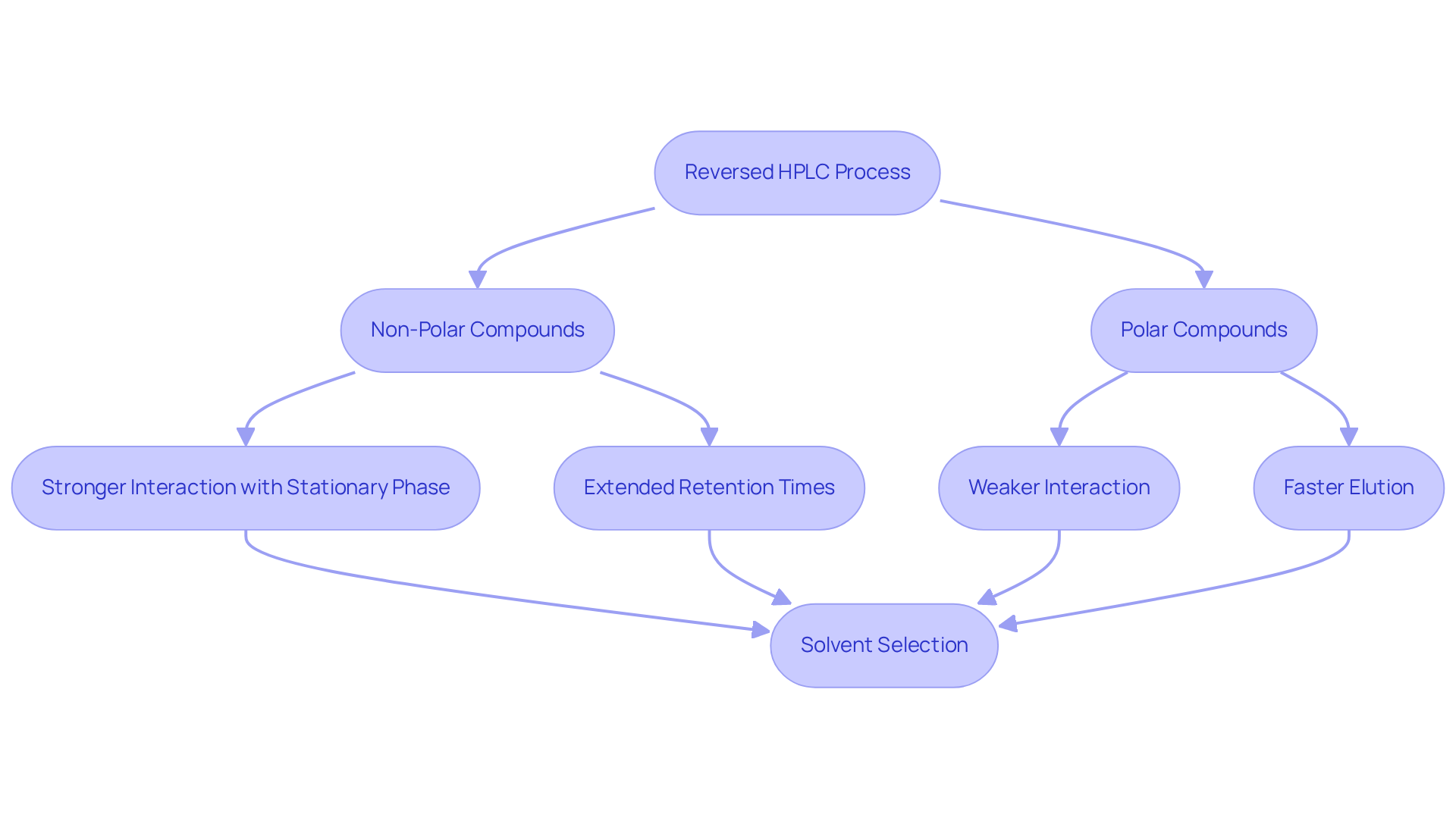
Reversed HPLC stands as a pivotal analytical method, adept at separating substances based on their hydrophobicity. This technique employs a non-polar stationary phase alongside a polar mobile phase, establishing a foundation for effective separation. The core principle of RP-HPLC dictates that non-polar compounds exhibit stronger interactions with the stationary phase, resulting in extended retention times, whereas polar compounds elute more swiftly. Such a separation mechanism, like reversed HPLC, is instrumental in the analysis of diverse substances, encompassing pharmaceuticals, environmental samples, and biological materials.
Furthermore, the selection of solvents—typically a blend of water and organic solvents like methanol or acetonitrile—is critical, significantly influencing the efficiency and selectivity of the separation process. By understanding these fundamental aspects, researchers can optimize their analytical strategies for superior results.
Explore the History and Evolution of Reversed HPLC
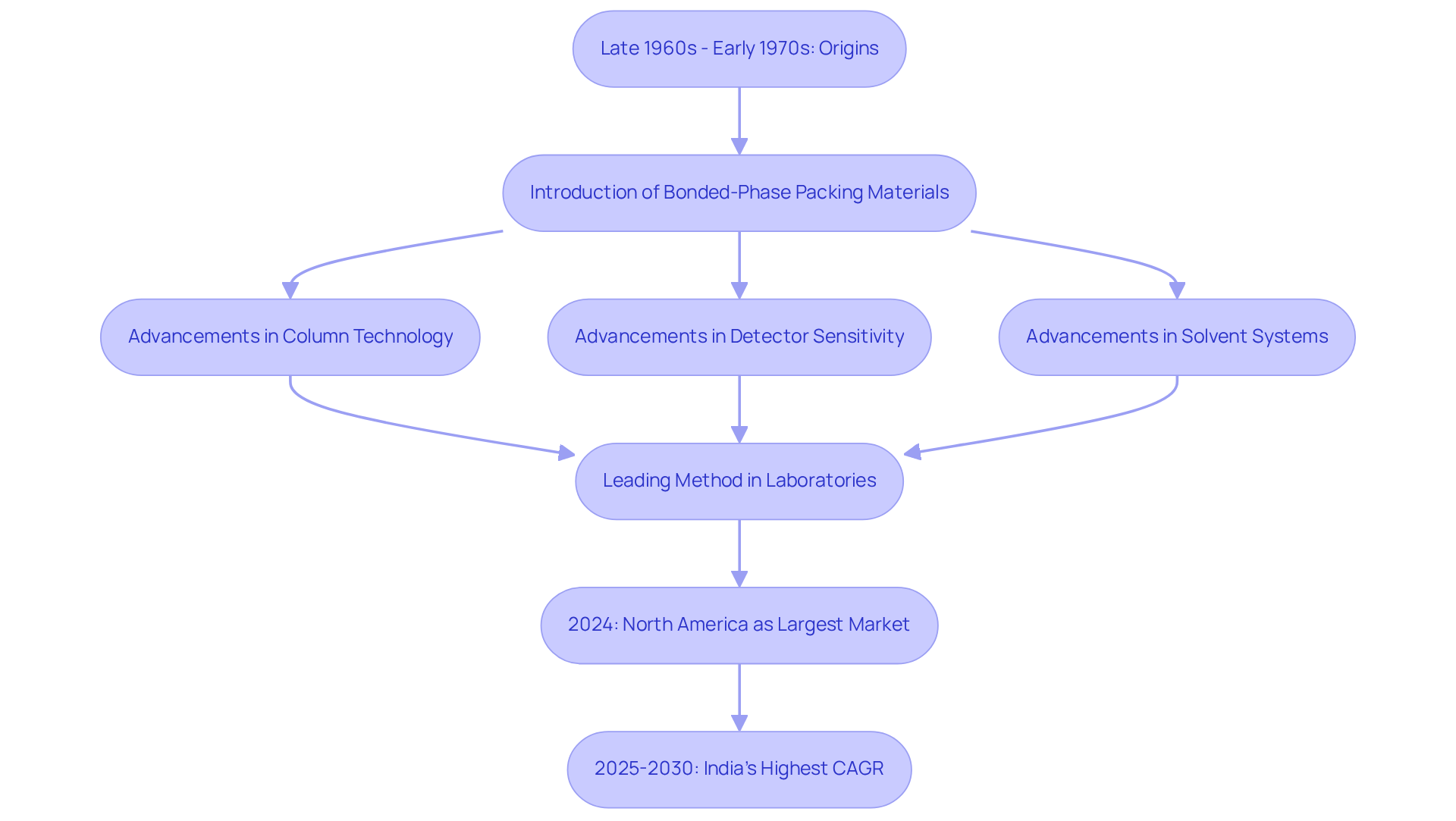
The origins of reversed HPLC can be traced back to the late 1960s and early 1970s, when researchers began to explore the limitations of conventional liquid separation techniques, including reversed HPLC. A pivotal advancement in this field was the introduction of bonded-phase packing materials, such as silica gel modified with hydrocarbon chains. This innovation catalyzed the development of reversed HPLC separation techniques, which quickly gained popularity due to their enhanced reproducibility and versatility compared to normal-phase methods.
Over the decades, significant advancements in column technology, detector sensitivity, and solvent systems have further augmented the capabilities of reversed HPLC, establishing it as the leading method of analysis in contemporary laboratories. Notably, North America emerged as the largest revenue-generating market for reversed HPLC columns in 2024, underscoring its critical role within the industry. Today, reversed HPLC finds application in various sectors, including pharmaceutical analysis and environmental monitoring, serving end-users such as pharmaceutical and biotech companies, along with academic and research institutions. This widespread utilization underscores its importance in scientific research.
The historical evolution of reversed HPLC separation methods, particularly the transition from hydrophilic unmodified silica to alkyl chain-modified surfaces, has facilitated the effective isolation of diverse substances, including amino acids, proteins, and nucleic acids. This progression solidifies reversed HPLC's status as a fundamental approach in analytical liquid methods. Furthermore, projections indicate that India is expected to register the highest CAGR in the reversed HPLC columns market from 2025 to 2030, highlighting emerging trends and opportunities in this dynamic field.
Examine Applications of Reversed HPLC in Scientific Research
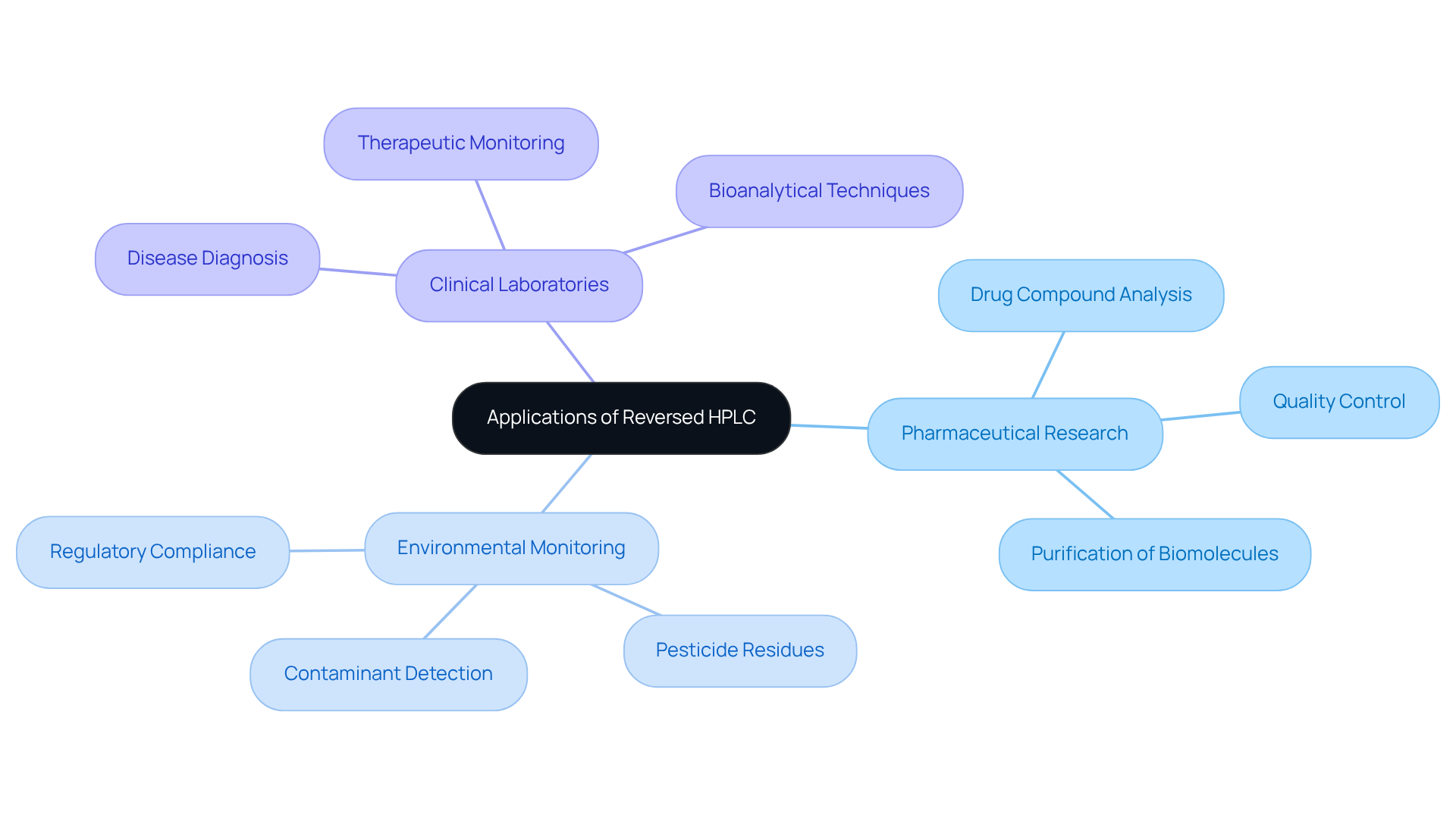
Reversed HPLC stands as a cornerstone method in numerous scientific domains, renowned for its exceptional ability to separate complex mixtures with precision. Within pharmaceutical research, reversed HPLC is indispensable for analyzing drug compounds, ensuring rigorous quality control and compliance with regulatory standards. This technique not only facilitates the characterization of metabolites but also aids in the purification of biomolecules, such as proteins and peptides, using reversed HPLC, which are vital for drug development and therapeutic applications.
Environmental scientists leverage reversed HPLC to identify and quantify contaminants in water and soil samples, significantly enhancing environmental monitoring efforts. This method proves particularly effective in detecting a diverse array of contaminants, including pesticides, herbicides, and industrial chemicals, thereby ensuring adherence to safety regulations. For instance, reversed HPLC has been successfully employed in research to monitor pesticide residues in food products, underscoring its critical role in safeguarding public health.
In clinical laboratories, reversed HPLC is essential for the analysis of biological samples, playing a crucial role in disease diagnosis and treatment monitoring. Approximately 70% of clinical laboratories utilize reversed HPLC for biological sample analysis, underscoring its importance in healthcare. The technique's remarkable resolving power and sensitivity enable the detection of trace levels of medications and metabolites, which are essential for pharmacokinetic studies and therapeutic drug monitoring. Notably, bioanalytical techniques developed for compounds such as curcumin and quercetin exemplify the practical applications of reversed HPLC in clinical settings.
The versatility and reliability of reversed HPLC make it an indispensable tool in both research and applied sciences, propelling advancements in pharmaceutical development, environmental safety, and clinical diagnostics. However, challenges such as pH range limitations and the presence of unreacted silanol groups must be addressed to enhance its applications. As highlighted by AltaBioscience, 'In reversed HPLC analysis, compounds are partitioned based on their difference in hydrophobicity,' emphasizing the foundational principles of this technique and its pivotal role across various scientific fields.
Conclusion
Reversed HPLC stands as a pivotal analytical technique that harnesses the principles of hydrophobicity to proficiently separate a diverse array of substances. By integrating a non-polar stationary phase with a polar mobile phase, this method facilitates the differentiation of compounds based on their interactions, thereby enhancing analytical precision. The importance of reversed HPLC transcends mere separation; it is a fundamental instrument across various scientific disciplines, including pharmaceuticals, environmental science, and clinical diagnostics.
This article has delved into essential facets of reversed HPLC, tracing its historical evolution from the late 1960s to its contemporary applications across multiple sectors. The advancement of this technique, marked by innovations in column technology and solvent systems, has established its reputation as a leading method within laboratories globally. Its applications in the analysis of drug compounds, environmental contaminants, and biological samples underscore its versatility and critical role in ensuring safety and efficacy across diverse fields.
The significance of reversed HPLC is profound, as it continues to propel innovations in scientific research and practical applications. Emerging trends reveal a burgeoning market for reversed HPLC, particularly in regions such as India, presenting an opportunity for researchers and practitioners to harness this technique for superior analytical outcomes. By embracing the principles and advancements of reversed HPLC, stakeholders can not only optimize existing methodologies but also pave the way for future breakthroughs in analytical science.




