Overview
Titration stands as a cornerstone in chemistry laboratories, offering a precise method for determining the concentration of unknown solutions through controlled reactions with a titrant of known strength. Its significance extends across various fields, including pharmaceuticals and environmental testing. The accuracy, cost-effectiveness, and ability to ensure compliance with safety standards and quality control make titration an indispensable tool in scientific practice. By understanding the pivotal role of titration, laboratories can enhance their analytical capabilities and uphold rigorous quality assurance protocols.
Introduction
Titration is a cornerstone of analytical chemistry, facilitating the precise measurement of unknown concentrations through systematic reactions with known titrants. This essential technique supports various industries, including pharmaceuticals and environmental science, while also prompting critical inquiries regarding its comparative advantages over alternative analytical methods. As laboratories increasingly strive for accuracy and efficiency, one must consider: what makes titration the preferred choice despite the rise of advanced technologies?
Understanding Titration: Principles and Applications
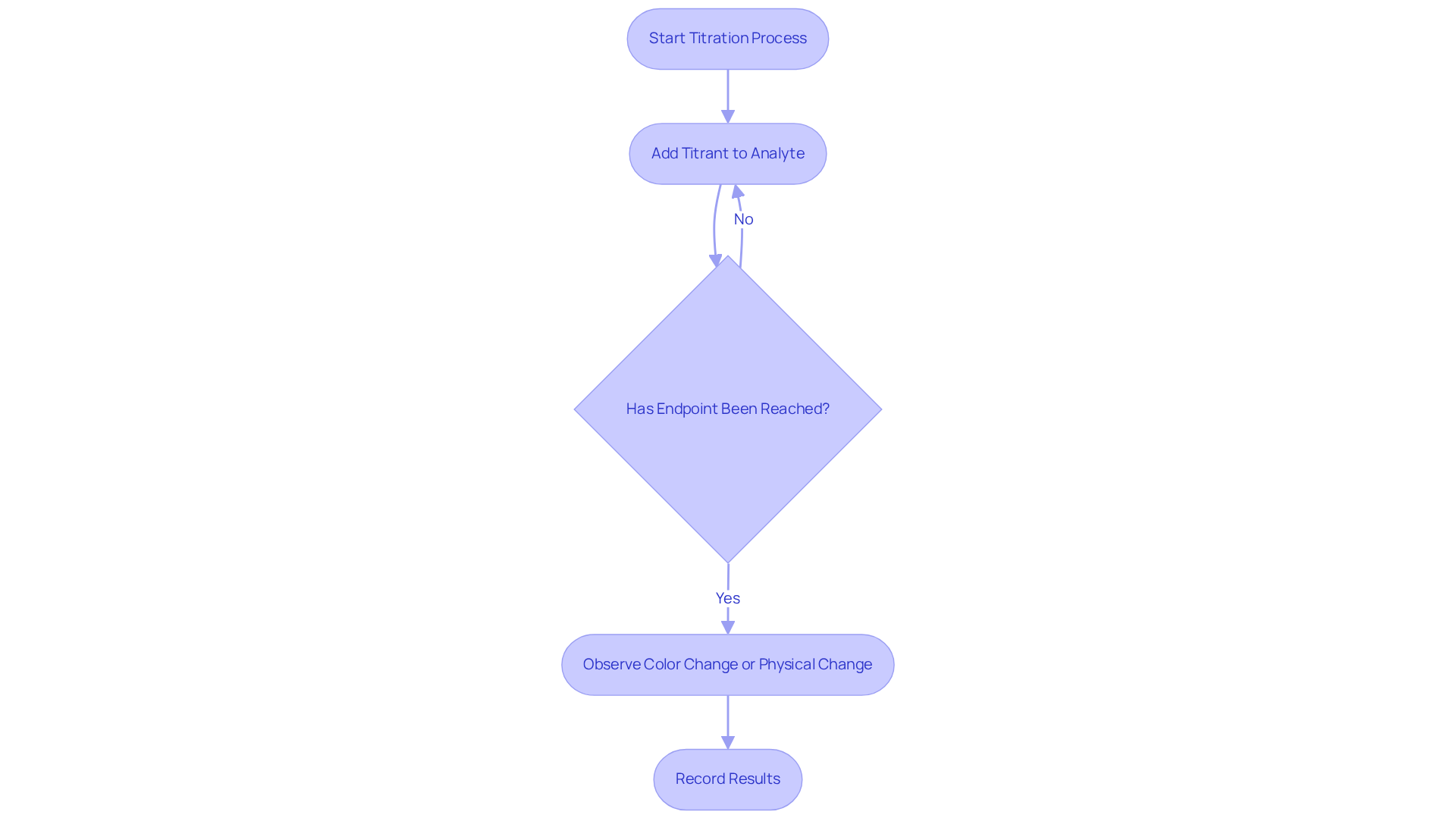
Titration is a pivotal quantitative analytical method, which of the following is the main reason to use titration in chemistry, as it is utilized to ascertain the strength of an unknown liquid through its reaction with a titrant of known strength. This meticulous process entails the gradual addition of the titrant to the analyte until the reaction reaches its endpoint, which of the following is the main reason to use titration in chemistry, often signaled by a distinct color change or a measurable alteration in a physical property. In the context of diverse fields such as pharmaceuticals, environmental testing, and food quality control, one may ask which of the following is the main reason to use titration in chemistry? In pharmaceutical laboratories, for instance, this process is indispensable for determining the concentration of active ingredients in drug formulations, which raises the question: which of the following is the main reason to use titration in chemistry, thereby ensuring compliance with stringent regulatory standards.
JM Science Inc. provides an extensive array of high-quality titrators, Karl Fischer reagents, and HPLC products, all designed to enhance the precision and efficacy of measurement processes. These products are meticulously crafted to meet the rigorous demands of laboratory environments, ensuring that laboratories can achieve accurate results in their testing efforts. Moreover, this analytical technique plays a crucial role in environmental chemistry, which of the following is the main reason to use titration in chemistry, as it is employed to scrutinize water samples for pollutants and contaminants, thus bolstering sustainability initiatives.
With the sophisticated measurement solutions offered by JM Science, laboratories are empowered to attain the highest standards of accuracy in their testing endeavors, reinforcing the critical role that precise analytical methods play in scientific research and quality assurance.
Comparing Titration with Alternative Analytical Techniques
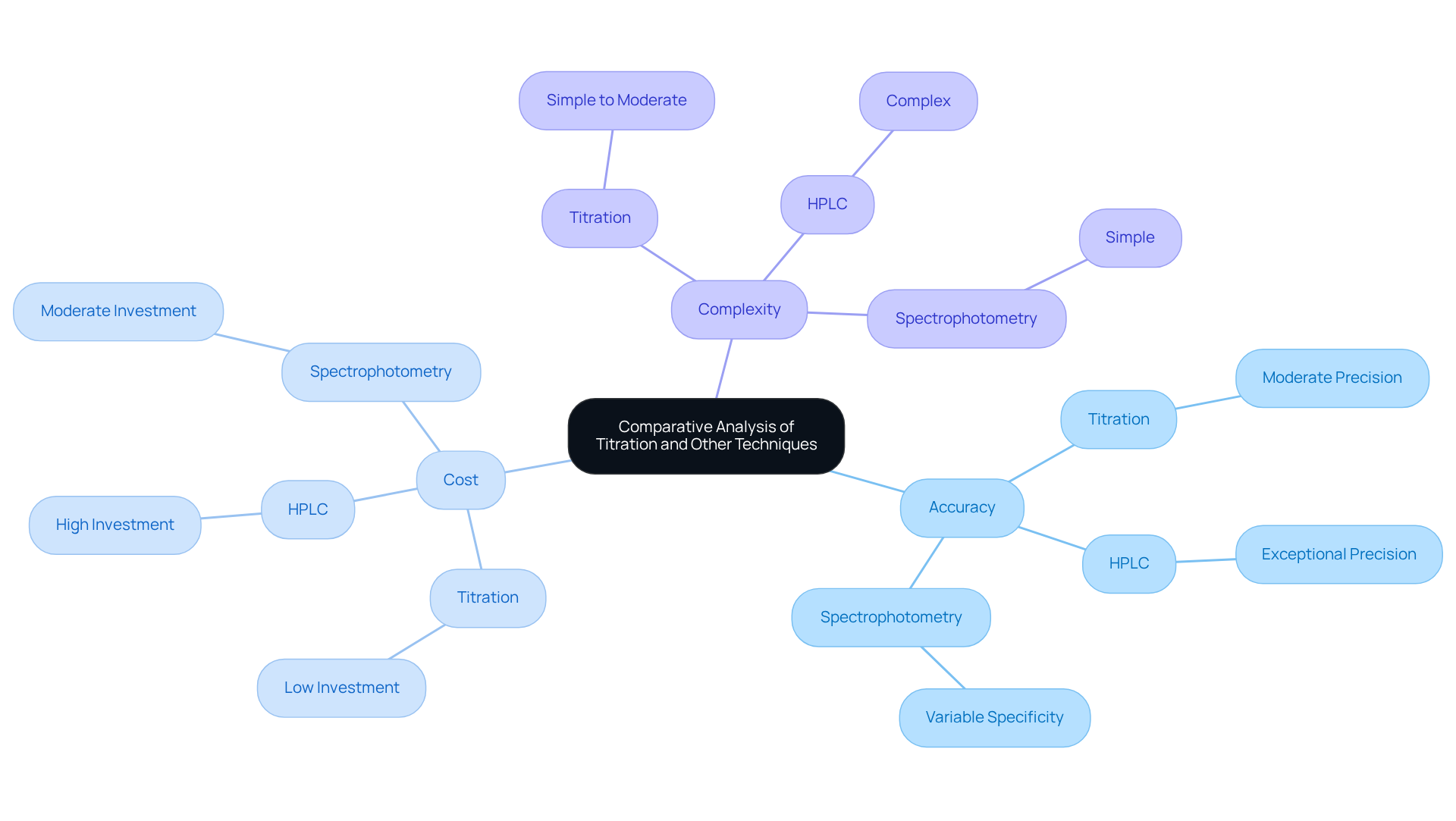
When contrasting this method with other analytical techniques, several critical factors emerge, including accuracy, cost, and complexity. High-Performance Liquid Chromatography (HPLC) is frequently favored for its exceptional ability to separate and analyze complex mixtures with remarkable precision. However, it necessitates a substantial investment in both equipment and training. In contrast, this analytical method is generally more cost-effective and simpler to perform, making it accessible for a wide range of laboratories.
Spectrophotometry, another widely used technique, measures the absorbance of light by a solution to determine the concentration of a substance. While it can be quicker than the discussed method, it may not provide the same level of specificity for certain reactions. Ultimately, the choice between these methods hinges on the specific requirements of the analysis, such as the nature of the sample and the desired accuracy.
Evaluating the Pros and Cons of Titration in Chemistry
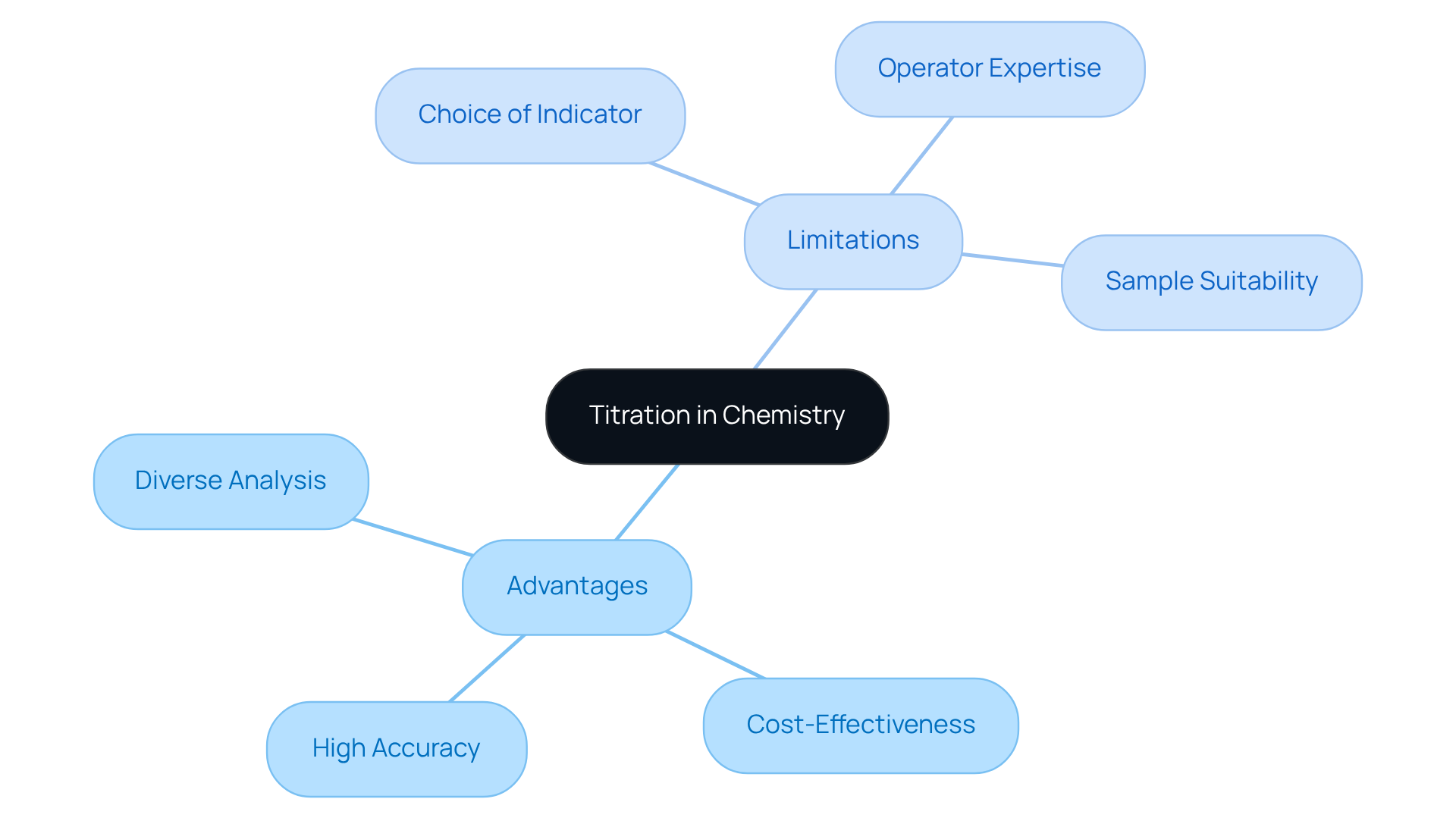
Among the various benefits of titration, which of the following is the main reason to use titration in chemistry? These advantages include high accuracy, cost-effectiveness, and the capability to analyze a diverse array of substances. This technique is particularly valuable for determining the concentration of acids, bases, and other reactive compounds.
However, it is essential to acknowledge the limitations inherent in the process. The precision of titration can be influenced by factors such as:
- The choice of indicator
- The operator's expertise
- The presence of interfering substances
Additionally, measuring may not be suitable for every type of sample, particularly those that are pigmented or cloudy, which can obstruct endpoint detection. While manual measurement remains prevalent, it is often time-consuming and susceptible to human error. Consequently, there has been an increasing reliance on automated dosing systems, such as those offered by JM Science. These systems enhance accuracy and effectiveness, positioning themselves as crucial instruments for pharmaceutical lab managers.
Identifying Ideal Scenarios for Titration Use
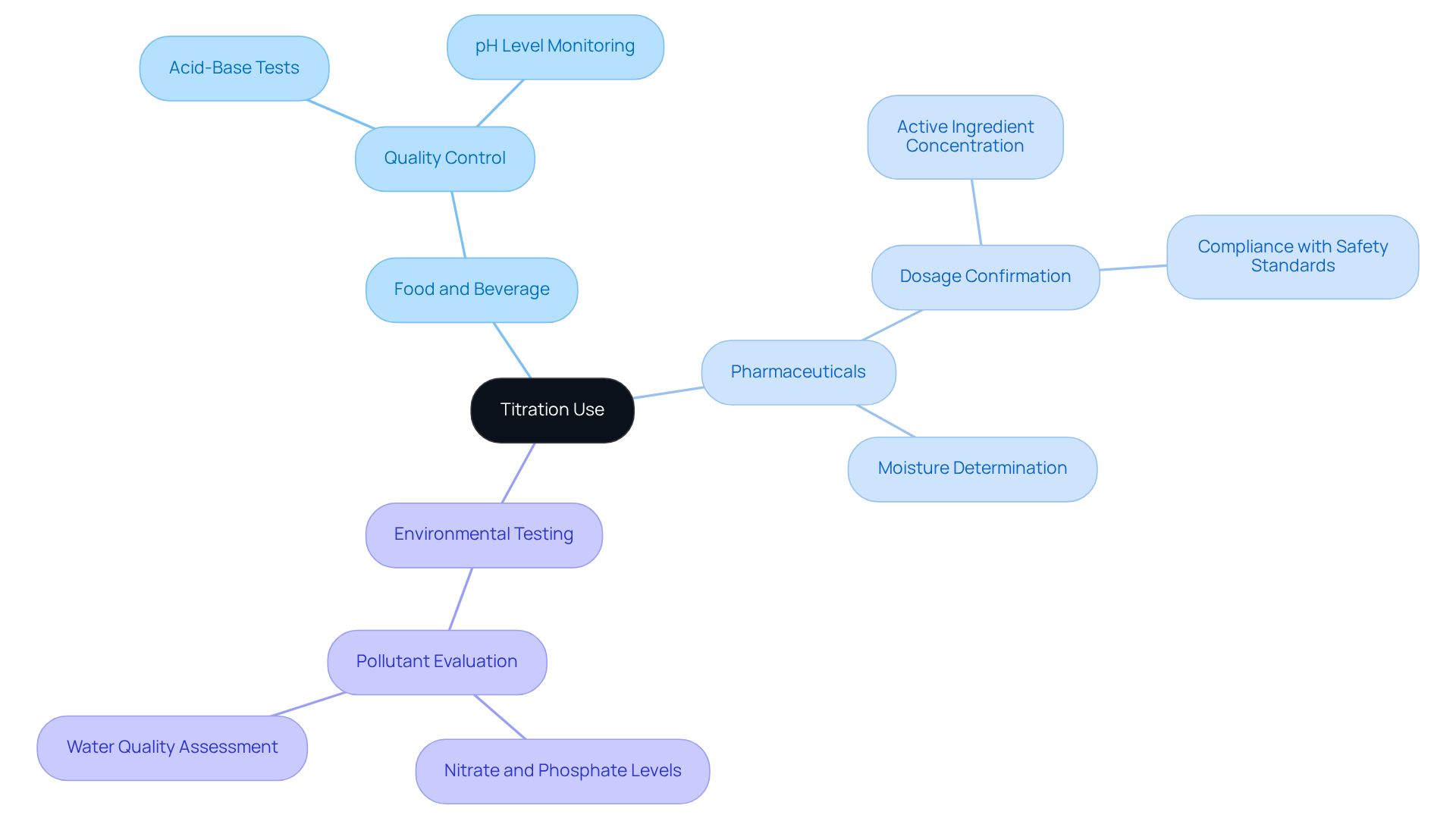
Titration is vital in scenarios requiring precise concentration measurements, and JM Science Inc. offers premium titrators that elevate this analytical technique. In the food and beverage sector, acid-base tests are crucial for quality control, ensuring specific pH levels are maintained for product safety and quality. For instance, the transition of bromothymol blue from yellow in acidic environments (pH < 6) to blue in alkaline conditions (pH > 7.6) underscores the importance of monitoring pH levels, as fluctuations can affect product integrity.
In pharmaceutical laboratories, accurate measurement is essential for confirming the correct dosage of active ingredients in medications, ensuring adherence to safety standards. The integrity of pharmaceutical products hinges on rigorous analytical techniques, with this process being paramount, supported by JM Science's advanced solutions, including Karl Fischer reagents for moisture determination.
Routine checks of raw materials and final products are crucial for maintaining the integrity of pharmaceutical formulations, as the equivalence point is achieved when the moles of reagent equal the moles of the analyte, typically around pH 7 for strong acid-strong base reactions.
Furthermore, quantitative analysis methods are employed in environmental testing to evaluate pollutant concentrations in water samples, revealing levels of nitrates and phosphates that are essential for monitoring water quality and ecological health. However, it is important to recognize that this analytical method may not be suitable for complex mixtures or samples that require rapid analysis, where techniques such as chromatography, for which JM Science also provides high-performance solutions, may prove more effective.
Common sources of error in this technique include human errors, inappropriate indicators, and contamination, which must be considered when implementing it. Ultimately, the decision to utilize titration should align with the specific analytical requirements and the nature of the samples being analyzed.
Conclusion
Titration serves as a cornerstone of analytical chemistry, crucial for accurately determining the concentration of unknown solutions through its precise methodology. This technique supports a diverse array of applications across multiple fields, including pharmaceuticals, environmental science, and food quality control, while underscoring the paramount importance of accuracy and reliability in laboratory settings.
The article elucidates the fundamental principles of titration, drawing comparisons with other analytical methods such as HPLC and spectrophotometry. It delineates the advantages of titration, including its cost-effectiveness and straightforward implementation, while also addressing its limitations, such as susceptibility to human error and the influence of external factors on results. Furthermore, the significance of utilizing advanced titration solutions from companies like JM Science is emphasized, demonstrating how modern technology can enhance the efficacy of this analytical process.
Ultimately, grasping the role of titration in chemistry is vital for laboratory professionals striving to uphold high standards of quality and accuracy. By recognizing when and how to employ titration effectively, researchers and technicians can ensure the integrity of their analyses, thereby contributing to advancements in scientific research and product safety. Embracing best practices in titration not only streamlines laboratory operations but also reinforces the essential nature of this analytical technique in achieving reliable results.




