Overview
The article presents a comprehensive overview of the numerous benefits of PVDF filters in pharmaceutical laboratories. These filters play a crucial role in enhancing operational efficiency, maintaining sterility, and ensuring compliance with stringent regulatory standards.
Specifically, the advantages of PVDF filters include:
- Remarkable chemical resistance
- Exceptional durability
- Ease of cleaning
- Versatility
Collectively, these features contribute significantly to improved operational effectiveness and heightened product safety within drug manufacturing processes. By understanding the critical role of high-quality scientific instruments, stakeholders can make informed decisions that bolster laboratory performance.
Introduction
In the realm of pharmaceutical laboratories, the pursuit of purity and safety is paramount. JM Science's PVDF filters stand at the forefront of this critical endeavor. Designed to meet the stringent demands of the industry, these filters excel in applications ranging from sterile filtration to sample preparation. They ensure that every drop of pharmaceutical product maintains its integrity.
With precise pore size distribution and remarkable chemical resistance, PVDF filters not only enhance operational efficiency but also play a vital role in compliance with regulatory standards. As the pharmaceutical filtration market continues to expand, the innovative capabilities of these filters promise to redefine benchmarks for quality and reliability in laboratory settings.
JM Science PVDF Filters: Enhanced Filtration Solutions for Pharmaceutical Labs
JM Science presents a specialized selection of membrane products tailored for laboratory environments, meticulously crafted to meet the sector's stringent standards for efficiency and reliability. These devices excel in applications such as sterile separation and sample preparation, which are vital for preserving the integrity of medicinal products. The unique characteristics of a PVDF filter, particularly its precise and uniform pore size distribution, ensure consistent quality of separation, making it indispensable for maintaining product purity. As emphasized by Grand View Research, this specific pore size distribution is crucial for achieving reliable separation outcomes in medical applications.
In conjunction with effective filtration, the use of the AQ-300 Coulometric Karl Fischer Titrator and the AQV-300 Volumetric Karl Fischer Titrator is essential for drug and medicine testing, particularly in compliance with the Japanese Pharmacopoeia. These titrators facilitate accurate moisture content assessment, a critical factor in ensuring the quality and safety of medicinal products. The AQ-300 boasts a measurement range of 1 ppm to 100% water content, while the AQV-300 is designed for volumetric titration with a similar range, enhancing their applicability in medical contexts. The drug purification market is projected to experience significant growth, with the research and development scale segment anticipated to rise at a CAGR of 11.45% throughout the forecast period. This growth is driven by the increasing demand for efficient purification solutions, underscoring the pivotal role of JM Science's PVDF filter membranes in eliminating particles and impurities from pharmaceutical liquids. In 2023, the final product processing segment dominated the market, highlighting the importance of effective purification in ensuring the safety and quality of finished products, in alignment with industry standards.
The practical applications of JM Science's polymer membranes illustrate their effectiveness in sterile separation processes, where the removal of impurities is paramount. By incorporating these mechanisms, laboratories can enhance their operational efficiency while adhering to regulatory requirements. Expert opinions underscore the benefits of utilizing JM Science's polymer filters, noting their ability to significantly improve filtering processes. Additionally, the case study titled "Application Insights: Final Product Processing Segment" underscores the significance of liquid purification in guaranteeing product purity and safety, reinforcing the efficacy of JM Science's solutions.
Recent advancements in polymer membrane technology, such as enhanced membrane materials and improved separation techniques, further strengthen their application in the medical sector, ensuring that laboratories are equipped with state-of-the-art solutions. As the market share of specialized membranes continues to grow within the drug industry, JM Science remains committed to providing high-quality separation products that meet the evolving demands of laboratories, ultimately supporting advancements in drug research and manufacturing.
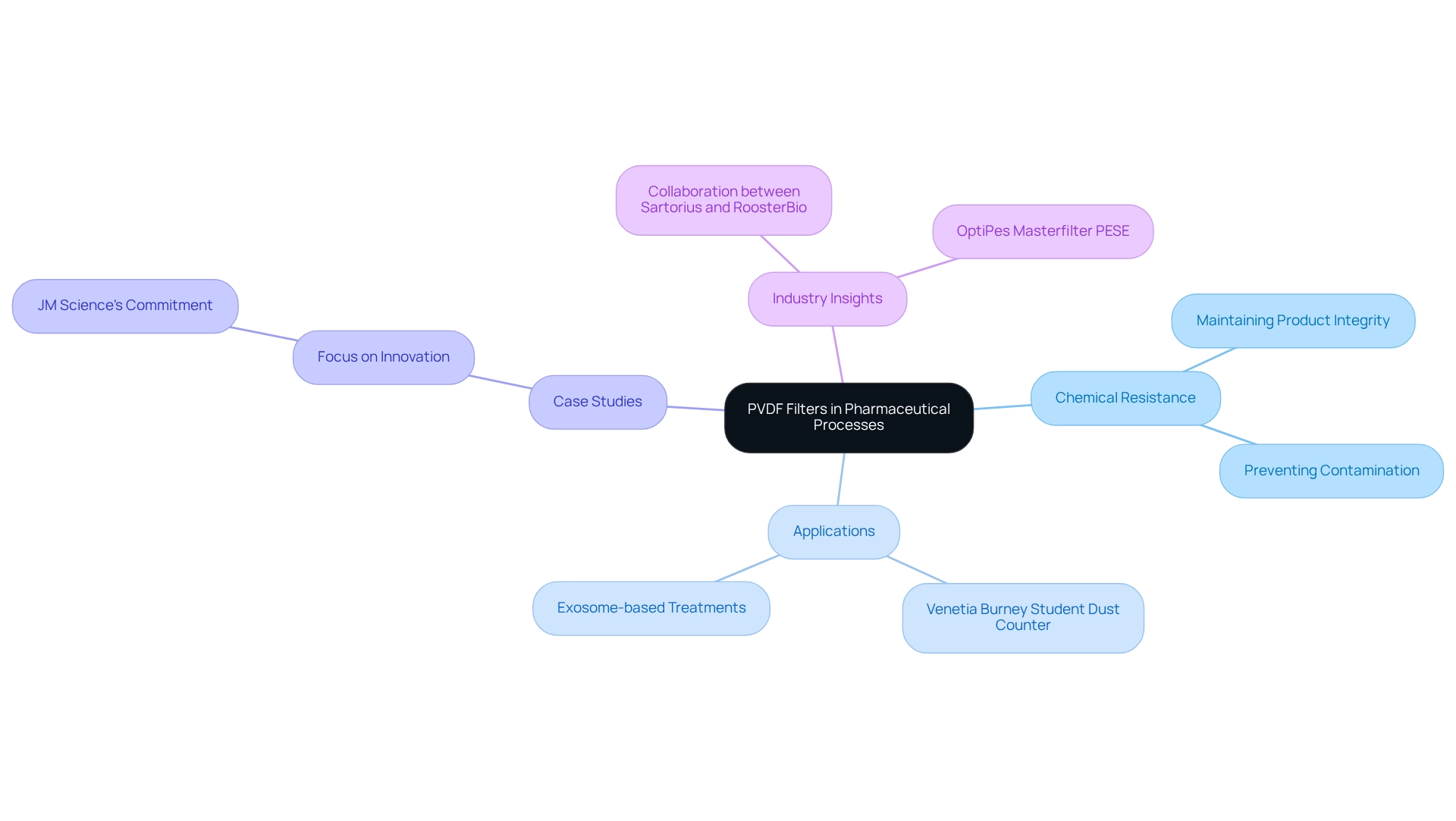
Chemical Resistance: Protecting Integrity in Pharmaceutical Processes with PVDF Filters
These membranes are recognized for their exceptional resistance to chemicals, making them indispensable in pharmaceutical procedures that involve harsh solvents and reagents. This remarkable resistance not only safeguards the integrity of the system itself but also protects the substances being processed, ensuring that no contaminants leach into the final product. Laboratories utilizing a pvdf filter can confidently manage a diverse array of chemicals without compromising the quality of their analyses or formulations.
Research indicates that a significant proportion of pharmaceutical procedures rely on specific membranes for their ability to handle aggressive solvents, underscoring their importance in maintaining chemical integrity. For instance, the Venetia Burney Student Dust Counter on the New Horizons spacecraft employs a specialized polymer, exemplifying its reliability even in extreme environments.
Industry leaders have noted that the chemical resistance of pvdf filter membranes surpasses that of many other materials, establishing them as a preferred choice in critical applications. This assertion is further supported by case studies demonstrating how pvdf filters have effectively preserved product integrity across various drug-related processes, reinforcing their role as a fundamental component in laboratory purification solutions. Moreover, the collaboration between Sartorius and RoosterBio to develop scalable production methods for exosome-based treatments highlights the innovative applications of these membranes in modern drug research.
By integrating specialized membranes into their workflows, pharmaceutical laboratories can enhance operational efficiency while upholding the highest standards of product quality. As one industry leader remarked, "The dependability and chemical integrity of these membranes are unparalleled, rendering them vital for any laboratory dedicated to precision and quality." This unwavering commitment to quality resonates with JM Science's dedication to innovation in scientific instruments, further solidifying the significance of polymer membranes in advancing research and healthcare.
Durability: Long-Lasting Performance of PVDF Filters in Rigorous Lab Environments
These membranes are engineered to withstand the demanding environments typical in medical research facilities. Their robust design guarantees consistent performance over extended periods, significantly minimizing the frequency of replacements. This durability not only boosts operational efficiency but also leads to substantial cost savings, making these membranes a prudent investment for laboratories prioritizing long-term reliability.
In rigorous testing conditions, such as those associated with the treatment of Murine Norovirus, specialized membranes have demonstrated remarkable log reductions of 3 to 7, underscoring their effectiveness in maintaining the high purity standards essential for medical applications.
Case studies reveal that laboratories utilizing a pvdf filter report enhanced durability, with many experiencing an average lifespan that exceeds expectations in pharmaceutical settings. JM Science Inc.'s diverse product range further bolsters this reliability, equipping labs with high-quality filtration solutions.
Expert insights highlight that the durability of these devices results in fewer workflow interruptions and lower overall maintenance costs, reinforcing their value in high-stakes environments. As noted by Brother Filtration, the use of specific membranes ensures that the processed liquid meets the required purity and quality standards, which is critical for compliance with stringent regulations.
By opting for specialized membranes, laboratories not only secure adherence to these criteria but also optimize their operational budgets while upholding rigorous public health standards through efficient disinfection.

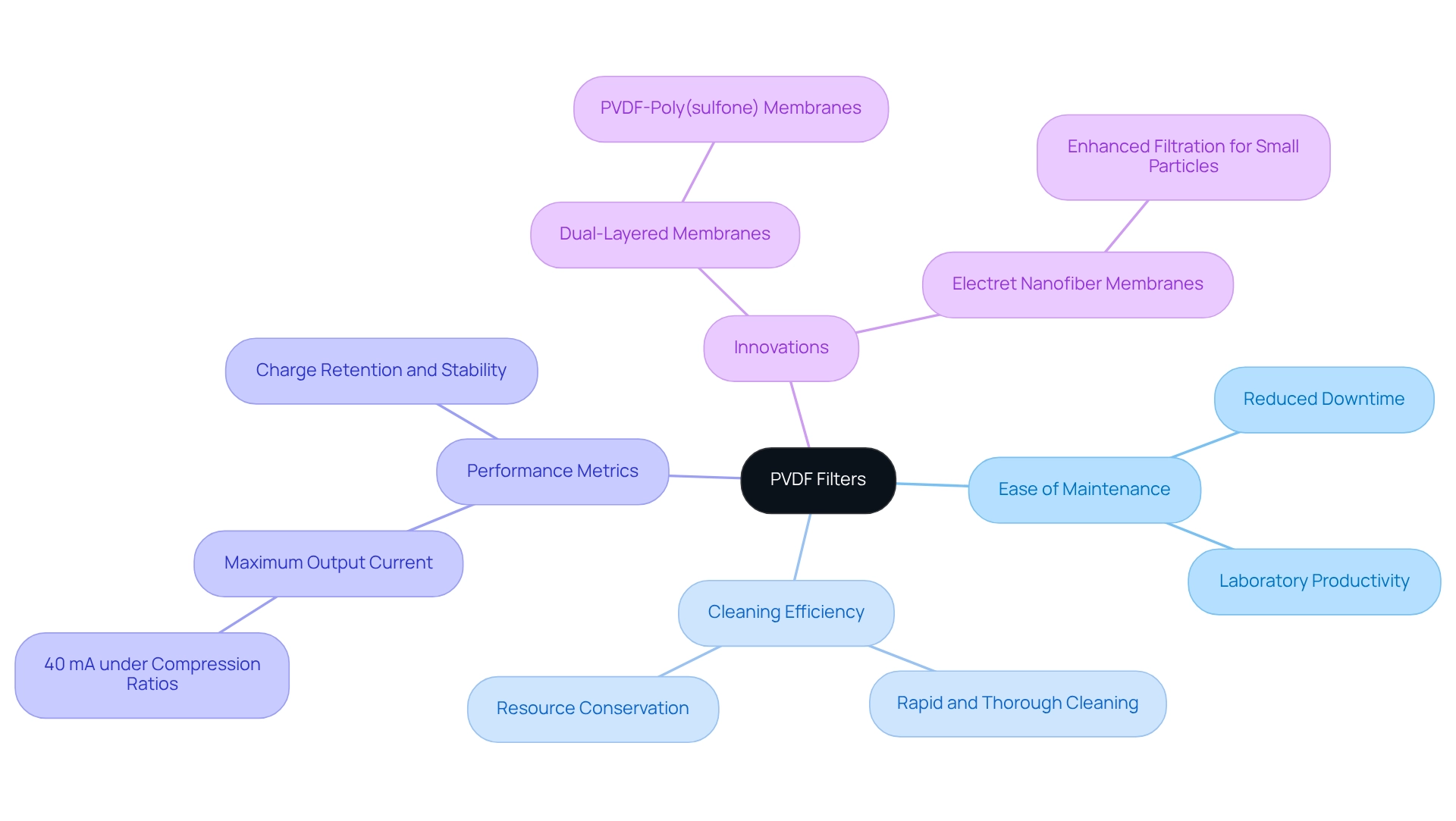
Ease of Cleaning: Streamlining Maintenance with PVDF Filters
These membranes stand out due to their exceptional ease of maintenance, making them an ideal choice for high-throughput laboratories where productivity is paramount. Their capacity for efficient cleaning and reuse significantly reduces downtime, enabling laboratories to maintain continuous operations. This capability not only streamlines maintenance procedures but also enhances overall productivity, allowing lab personnel to concentrate on critical tasks without the interruptions caused by frequent cartridge replacements. Data indicates that the pvdf filter membranes can achieve a maximum output current of 40 mA under compression ratios, showcasing their robust performance. Furthermore, in situ charging of polymer membranes results in prolonged charge retention and improved stability of filtration efficacy compared to traditional corona charging methods. This stability is particularly advantageous in pharmaceutical settings, where dependable performance is essential.
Case studies highlight the cleaning efficiency of these membranes, illustrating that their design facilitates rapid and thorough cleaning, which is vital in high-throughput environments. For instance, laboratories utilizing reusable membranes have reported significant time savings, leading to more effective workflow management. Research on electret nanofiber membranes for enhanced screening indicates that these electrically charged membranes improve performance, especially for small particles, further underscoring the efficacy of these devices in maintaining stringent cleanliness standards.
Insights from laboratory managers underline the importance of maintenance efficiency with these systems. Many have observed that the streamlined cleaning process not only conserves resources but also bolsters the reliability of filtration outcomes. This feedback accentuates the role of these membranes in upholding high cleanliness and operational efficiency standards in drug manufacturing facilities. Additionally, innovations such as dual-layered nanofiber membranes incorporating a pvdf filter and poly(sulfone) with improved permeation flux reflect ongoing advancements that enhance the performance of these membranes in laboratory contexts.
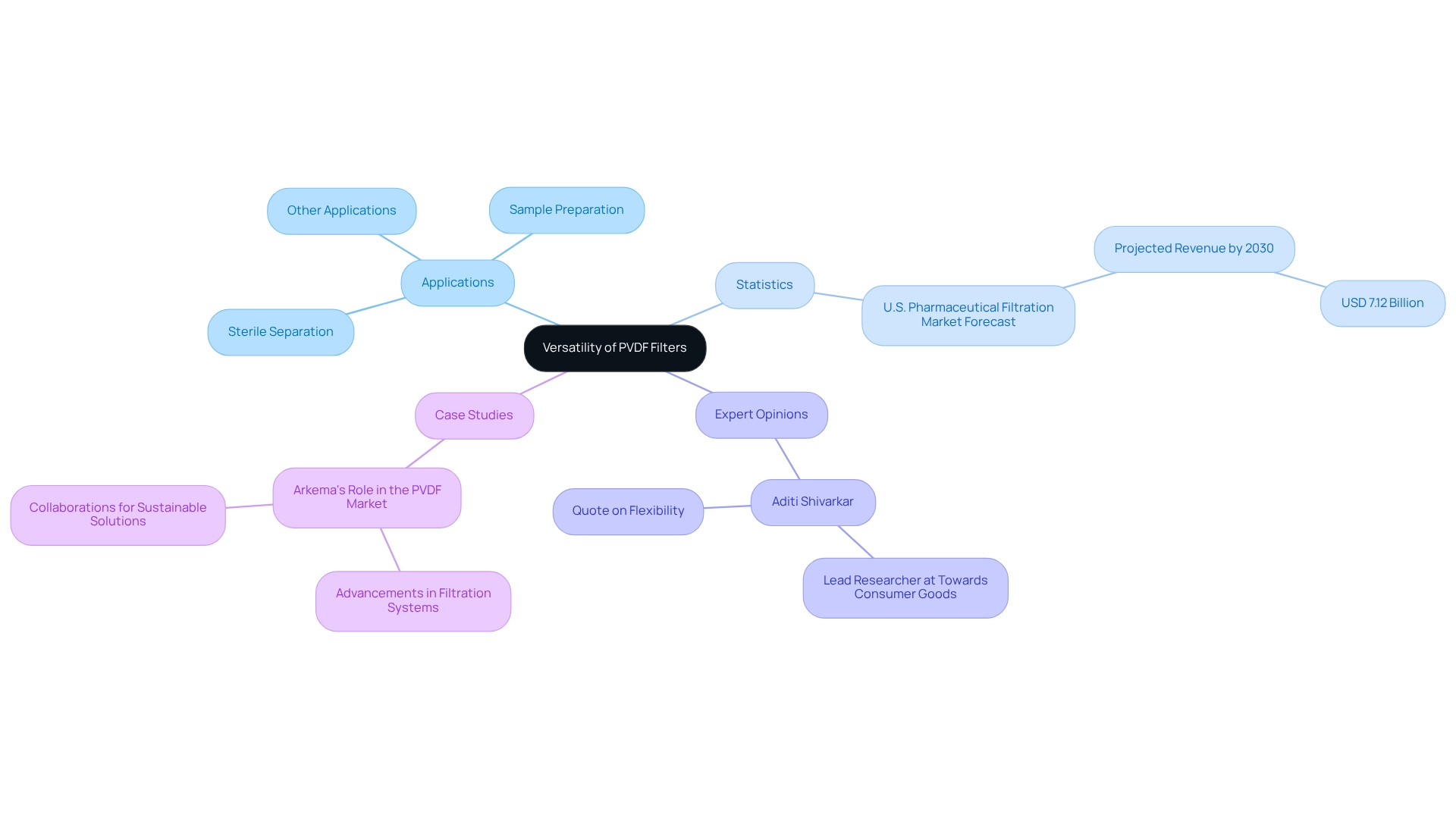
Versatility: Adapting PVDF Filters for Diverse Pharmaceutical Applications
These membranes, known as pvdf filters, are renowned for their adaptability, making them ideal for a variety of pharmaceutical applications, including:
- Sterile separation
- Sample preparation
- More
Their compatibility with a broad spectrum of solvents and reagents facilitates effective use in both aqueous and organic solutions. This flexibility empowers laboratories to employ polymer membranes across diverse processes, significantly enhancing operational efficiency and effectiveness.
Statistics indicate that a substantial number of laboratories are currently utilizing polymer membranes due to their robust performance across various applications. Notably, the U.S. drug purification sector is projected to reach USD 7.12 billion by 2030, underscoring the growing reliance on advanced purification technologies like specialized membranes, including the pvdf filter, which are essential for maintaining high standards in drug production.
Expert opinions highlight the exceptional adaptability of these membranes in medical settings. Aditi Shivarkar, a principal researcher at Towards Consumer Goods, notes that "the flexibility of purification technologies is essential for addressing the changing needs of the drug sector." This adaptability guarantees optimal performance across applications. For instance, in sterile separation, pvdf filters effectively remove impurities while safeguarding the integrity of sensitive samples. Furthermore, their resistance to high temperatures and abrasion renders them suitable for demanding chemical environments, thereby expanding their applicability.
Case studies illustrate the successful integration of polymer membranes in various drug manufacturing processes. For example, Arkema's advancements in fluoropolymer production have led to enhanced filtration systems that laboratories report using for activities ranging from drug formulation to quality control. This demonstrates the critical role that pvdf filters play in advancing drug research and manufacturing. Overall, the adaptability of these membranes positions them as a vital component in modern drug laboratories.
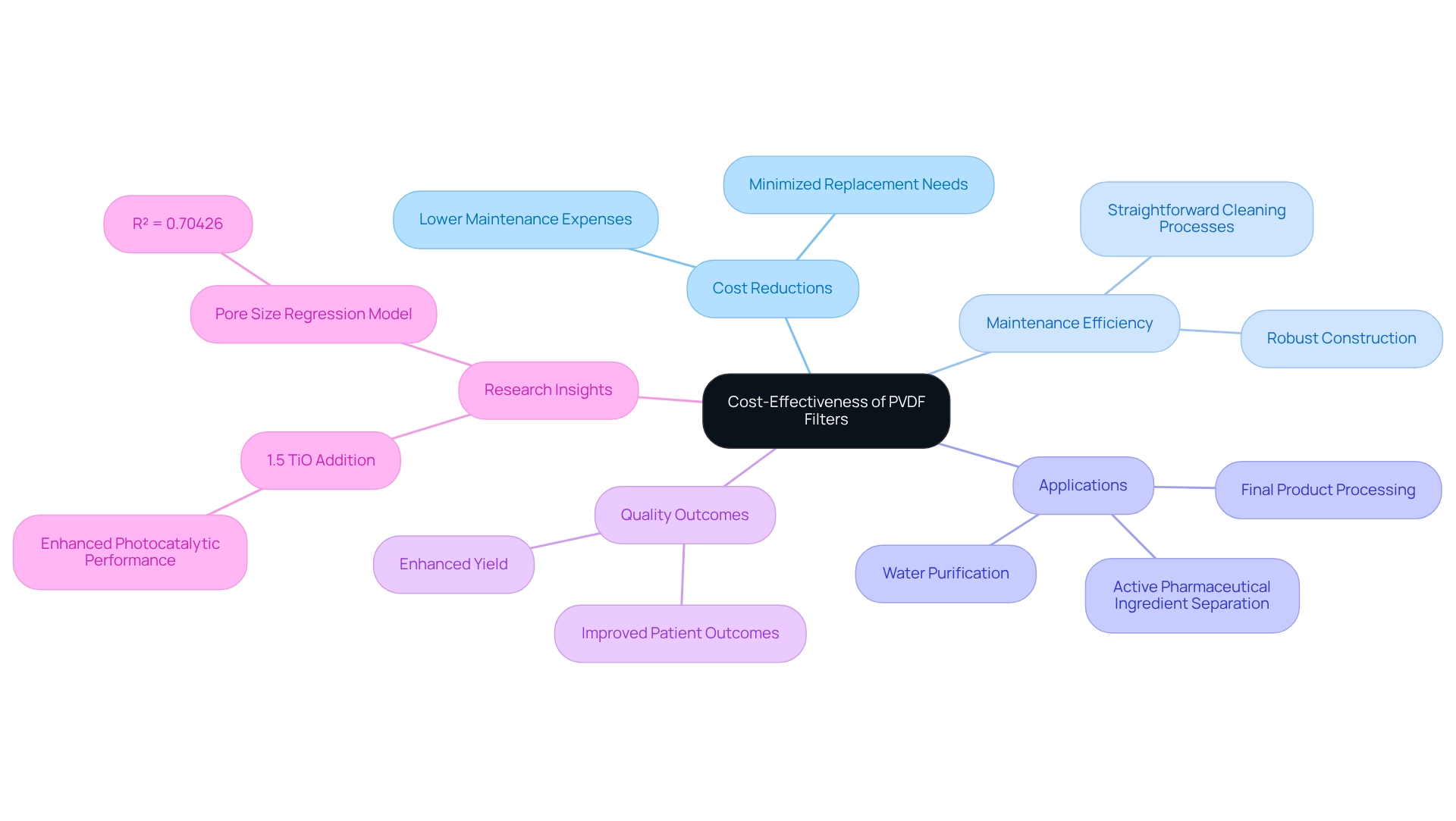
Cost-Effectiveness: Maximizing Value with PVDF Filters in Pharmaceutical Labs
Investing in polymer membranes presents a compelling opportunity for drug manufacturing facilities to achieve significant cost reductions. Their robust construction, coupled with straightforward cleaning processes, minimizes the need for frequent replacements and lowers maintenance expenses. The efficiency of the PVDF filter across various applications—including final product processing, active pharmaceutical ingredient separation, and water purification—not only reduces waste but also maximizes yield, thereby enhancing overall cost-effectiveness.
Notably, research indicates that the incorporation of 1.5% TiO into the dope solution can significantly enhance photocatalytic performance, refining purification processes further. Furthermore, the regression model for pore size boasts a predicted determination coefficient of R² = 0.70426, underscoring the effectiveness of these devices.
As S.A. Pillai emphasizes, "It is essential to reduce the negative interaction between the device and the drug product and thus provide the best to the patient and health care for which the end user needs to thoroughly comprehend their application, nature of the material being processed and specifics of the apparatus to better grasp the results of the separation process."
By selecting specialized membranes like the pvdf filter, laboratories can achieve high-quality results while adhering to budget constraints, ultimately leading to improved outcomes for both patients and healthcare professionals. To fully leverage the advantages of these membranes, lab managers should conduct a comprehensive assessment of their specific separation needs and consider integrating these membranes into their processes for enhanced efficiency and cost reductions.
Improved Product Yield: Enhancing Efficiency with PVDF Filters
The implementation of the pvdf filter is pivotal in enhancing product yield within drug manufacturing laboratories. With their high flow rates and low protein binding characteristics, pvdf filters ensure a greater recovery of the desired product during filtration processes. This remarkable efficiency not only boosts the overall productivity of the laboratory but also plays a crucial role in the successful development and production of medical products. By leveraging such advanced filtration technology, laboratories can achieve superior outcomes, thereby reinforcing their commitment to quality and innovation.
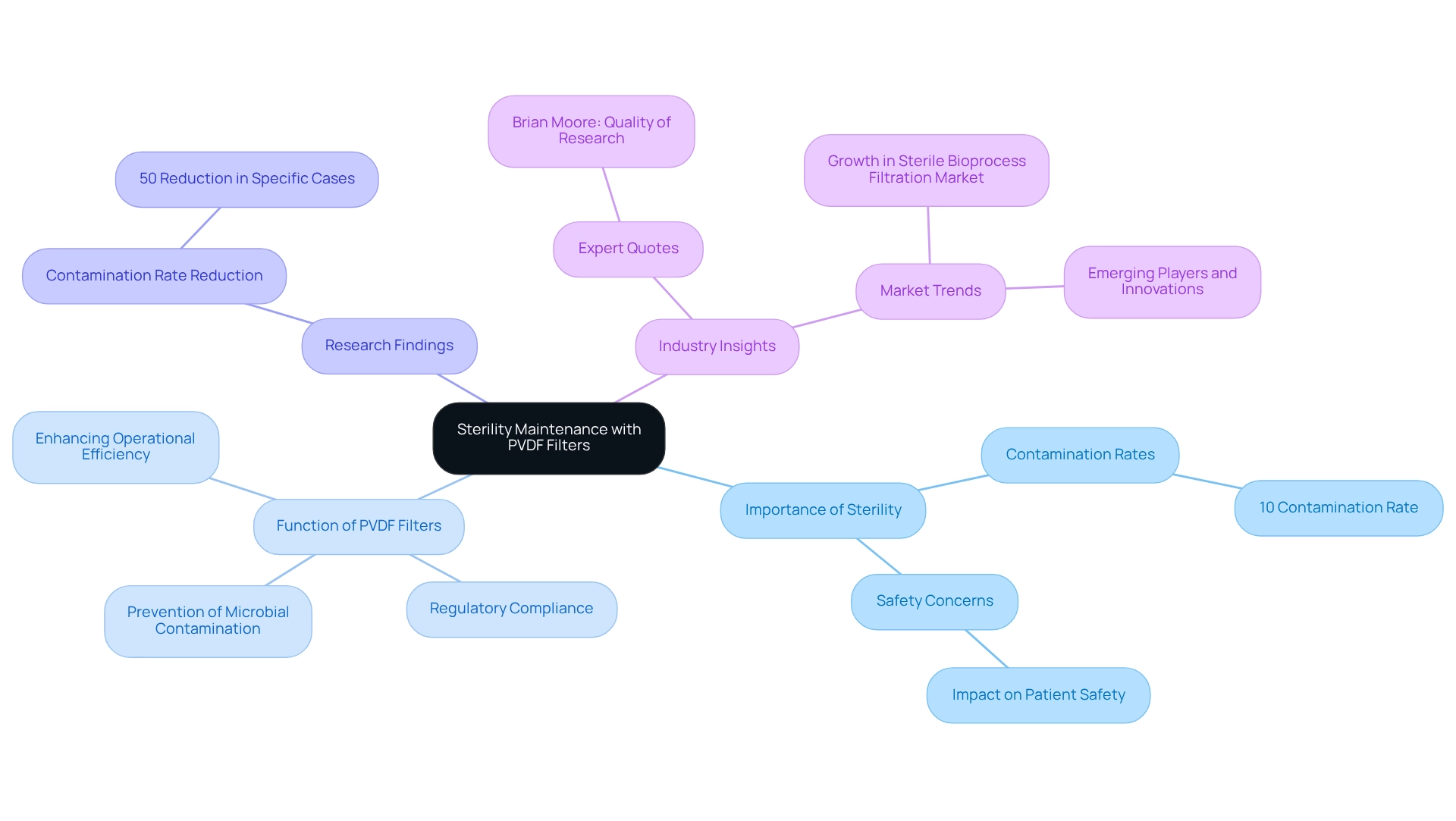
Sterility Maintenance: Ensuring Clean Processes with PVDF Filters
The pvdf filter plays a vital role in maintaining sterility within medical processes, effectively filtering out impurities to guarantee that products remain free from microbial contamination. This capability is critical in the production of sterile medications, where even the slightest contamination can result in serious safety concerns. Indeed, research shows that contamination rates in sterile pharmaceutical production can soar to 10%, significantly jeopardizing product integrity and patient safety, thereby highlighting the imperative for rigorous filtration methods.
Using a pvdf filter enables laboratories to maintain the highest cleanliness and safety standards. These devices not only prevent microbial contamination but also assist in fulfilling increasingly stringent regulatory requirements within the biopharmaceutical sector. As the sterile bioprocess purification market expands—driven by a rising demand for biologics and technological advancements—the reliability and effectiveness of the PVDF filter are distinguished.
Case studies further underscore the successful application of polymeric membranes across various pharmaceutical settings, showcasing their role in preserving sterility. For instance, companies have recorded significant reductions in contamination rates, with some achieving declines of up to 50% through the use of specific membranes in their production processes. Industry experts, such as Brian Moore, VP at NICCA USA, Inc., affirm that "the quality of research they have done for us has been excellent," emphasizing the critical nature of high-quality filtration solutions in mitigating microbial contamination.
In conclusion, the integration of polymer membranes not only enhances the cleanliness of pharmaceutical products but also contributes to overall operational efficiency, establishing them as an indispensable element in the quest for safe and effective pharmaceuticals.
Solvent Compatibility: Utilizing PVDF Filters Across Different Chemical Environments
These membranes exhibit exceptional compatibility with a broad spectrum of solvents, positioning them as an ideal choice for laboratories engaged in diverse chemical processes. Their robust chemical resistance ensures that these devices can endure exposure to various solvents without degradation, thereby maintaining optimal performance. This versatility empowers laboratories to deploy PVDF filters across multiple applications, from organic solvent separation to aqueous solutions, significantly enhancing operational flexibility.
In practical applications, PVDF filters have demonstrated remarkable effectiveness in solvent separation. A pertinent case study involved an automated decanting system for acetone and epoxy resin, where the demand for a precise filtration solution was critical. The implementation of SteriLUX devices facilitated efficient particulate removal and bioburden reduction, safeguarding the integrity of the solvents during the decanting process into 5 kg and 25 kg containers. This example illustrates how utilizing high-quality screens can bolster operational efficiency by mitigating contamination risks.
Moreover, compatibility data reveal that these membranes function reliably across various solvents, including aggressive substances commonly found in drug laboratories. Specifically, SteriLUX devices are designed to aid in particle removal and bioburden reduction in aqueous liquid streams, which is vital for maintaining compliance with stringent quality standards in drug manufacturing. By integrating specialized membranes, laboratories can adeptly navigate the complexities of solvent separation with a PVDF filter, ensuring both efficiency and reliability in their operations.
To fully leverage the benefits of these membranes, laboratory supervisors should consider incorporating them into their standard operating procedures for solvent processing, thereby enhancing both efficiency and compliance.
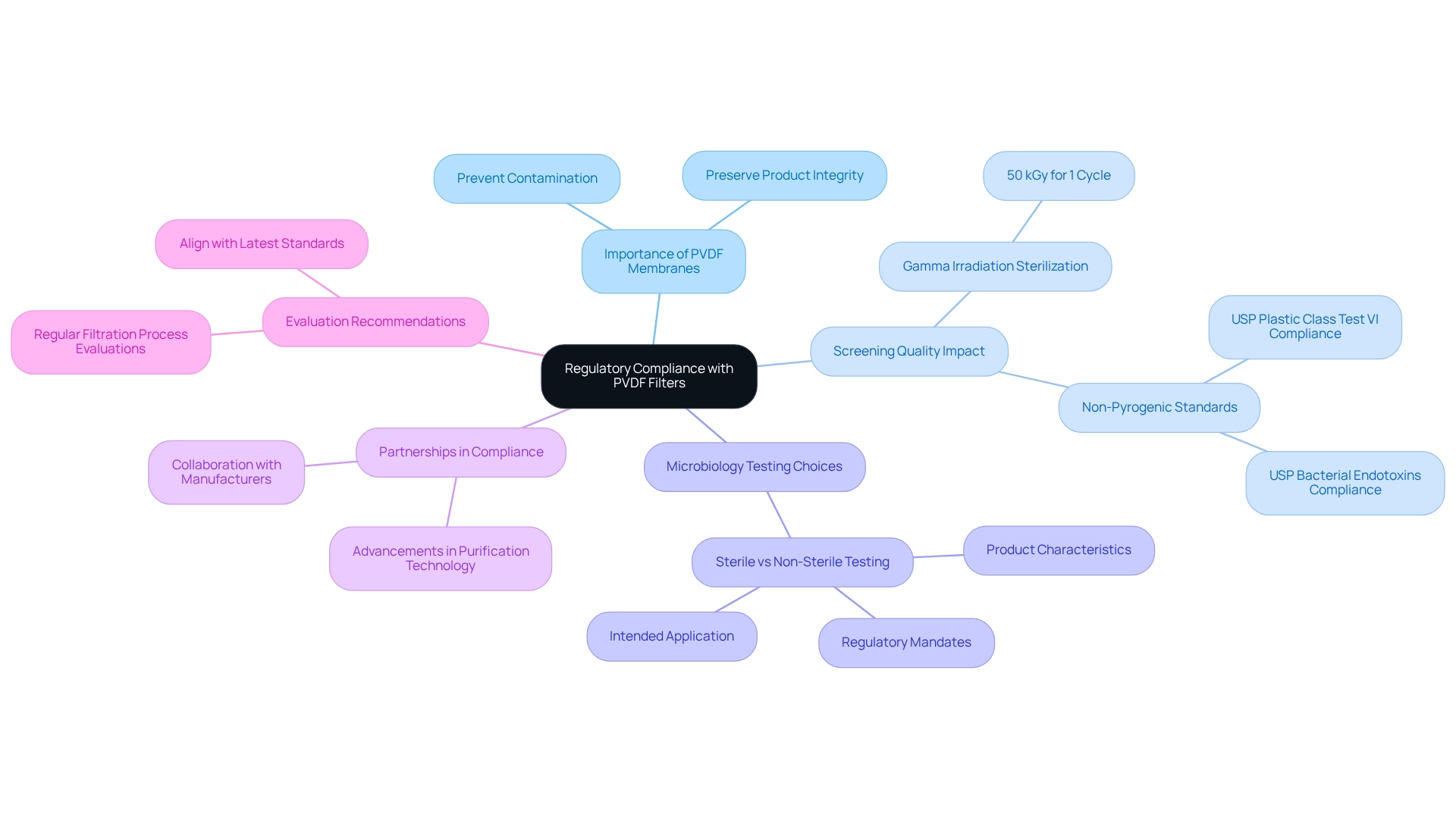
Regulatory Compliance: Meeting Standards with PVDF Filters in Pharmaceutical Labs
PVDF membranes are indispensable for laboratories striving to meet stringent regulatory compliance standards. These devices are meticulously designed to prevent the introduction of contaminants during the purification process, thus preserving the integrity and quality of pharmaceutical products. Adhering to regulatory requirements not only ensures product safety and efficacy but also fortifies trust with regulatory bodies and consumers alike.
The impact of screening quality on regulatory certifications is profound. For instance, gamma irradiation sterilization processes typically utilize 50 kGy for a single cycle, guaranteeing that screens fulfill the necessary sterility standards. Moreover, products like Virosart CPV devices are recognized as non-pyrogenic in accordance with USP Bacterial Endotoxins and comply with USP Plastic Class Test VI, further attesting to their reliability in meeting industry benchmarks.
Expert insights emphasize that the choice between sterile and non-sterile microbiology testing is largely dictated by the product's characteristics and intended application, alongside regulatory mandates. This highlights the critical nature of making informed decisions regarding microbiology testing to uphold the highest standards of safety and quality while aligning with regulatory frameworks. As Nelson Labs articulately states, "Our approach combines technical and analytical expertise, polymer knowledge, and understanding of regulatory requirements, all combined in a tailored approach for our customers."
By employing a pvdf filter, drug manufacturing facilities can effectively navigate the intricacies of regulatory compliance, ensuring their separation processes align with industry standards. The robust partnerships that JM Science Inc. maintains with leading manufacturers foster advancements in purification technology, further bolstering regulatory compliance. This unwavering commitment to quality not only enhances product safety but also positions laboratories as reliable partners within the pharmaceutical supply chain.
For lab managers contemplating the implementation of a pvdf filter, it is prudent to conduct regular evaluations of filtration processes and ensure that the pvdf filter selected aligns with the latest regulatory standards and technological innovations.
Conclusion
The integration of JM Science's PVDF filters into pharmaceutical laboratories marks a pivotal advancement in the quest for purity and safety in drug production. These filters are meticulously engineered to meet the industry's rigorous standards, excelling in applications such as sterile filtration and sample preparation. With precise pore size distribution and exceptional chemical resistance, they guarantee consistent filtration quality—an essential factor for preserving the integrity of pharmaceutical products.
Moreover, the durability and ease of cleaning associated with PVDF filters significantly enhance operational efficiency by minimizing maintenance costs and downtime. This optimization not only maximizes productivity but also empowers laboratories to concentrate on critical tasks without frequent disruptions. The versatility of these filters allows for their application across a wide array of processes, from organic solvent filtration to aqueous solutions, rendering them an invaluable asset for contemporary pharmaceutical research and production.
As the pharmaceutical filtration market continues to expand, propelled by escalating demands for effective filtration solutions, the importance of PVDF filters becomes increasingly pronounced. By complying with regulatory standards and facilitating the development of high-quality pharmaceuticals, these filters play a crucial role in ensuring the safety and efficacy of drug products. In conclusion, the adoption of JM Science's PVDF filters not only boosts product yield and operational efficiency but also reinforces the commitment to delivering safe and effective healthcare solutions. Laboratories prioritizing the integration of these advanced filtration technologies will undoubtedly position themselves at the forefront of innovation within the pharmaceutical industry.




