Overview
This article delineates ten pivotal benefits of employing 0.2 µm filters in pharmaceutical laboratories. These filters are essential for ensuring sterility, enhancing product integrity, and adhering to regulatory standards. By significantly reducing microbial contamination, they not only improve analytical accuracy but also yield cost savings. Consequently, their role in maintaining high-quality pharmaceutical practices and safeguarding patient safety cannot be overstated.
Introduction
In the realm of pharmaceutical manufacturing, the quest for purity and safety is paramount. At the forefront of this endeavor are 0.2 µm filters, essential guardians against microbial contamination. They ensure that every solution used in critical processes remains sterile. Recent advancements in filtration technology have heightened their significance, with studies revealing their ability to drastically reduce contamination rates while enhancing the integrity of pharmaceutical products. As the industry evolves, the reliance on these filters becomes increasingly vital, shaping the standards of quality and compliance that govern drug production and laboratory practices. This article delves into the multifaceted roles of 0.2 µm filters, exploring their impact on sterility, regulatory compliance, and overall product integrity across various applications within the pharmaceutical landscape.
JM Science 0.2 µm Syringe Filters: Precision for Sterile Applications
JM Science's 0.2 um filter syringe membranes are engineered for exceptional accuracy in sterile applications, effectively eliminating bacteria and other contaminants to ensure that solutions in drug laboratories remain free from microbial interference. Constructed from high-quality materials, these devices deliver reliable performance, making them indispensable for critical procedures such as cell culture and injectable medication preparation.
Recent advancements in purification technology using the 0.2 um filter have significantly improved their efficacy. Studies indicate a marked increase in filtration efficiency, which is vital for preserving the integrity of sensitive pharmaceutical formulations. A research project associated with Baylor College of Medicine and Texas Children's Hospital has shown that the use of a 0.2 um filter can reduce microbial contamination rates by over 90%, underscoring its importance in sterile applications.
Laboratory supervisors have noted that employing these devices not only optimizes workflows but also ensures compliance with stringent regulatory standards. As Thomas D. Horvath from the Department of Pathology and Immunology at Baylor College of Medicine remarked, 'The adjustment of our microbiological workflows to utilize the 0.2 um filter has significantly enhanced our laboratory's efficiency and safety.'
A case study from Embranes Solutions highlights how customized support in selecting the appropriate syringe component has led to improved separation processes, yielding better results across various applications. This emphasizes the critical role of expert guidance in achieving superior filtration outcomes.
As of 2025, the market share of the 0.2 um filter membranes in laboratories continues to grow, reflecting their vital role in maintaining sterile conditions. Recent market analysis reveals that the adoption of these devices has surged by 25% in the past year alone. Insights from laboratory supervisors consistently affirm the efficiency of these devices, reinforcing their status as an essential element in laboratories dedicated to precision and safety.
Ensuring Sterility: The Role of 0.2 µm Filters in Pharmaceutical Production
0.2 um filters are indispensable in drug manufacturing, effectively eliminating bacteria and other microorganisms from solutions. This ensures compliance with stringent sterility standards, which are vital for patient safety. The use of these filters transcends best practices; it is frequently mandated by regulatory bodies, underscoring their significance in upholding product integrity. Notably, over 80% of drug manufacturers utilize the 0.2 um filter to satisfy these sterility requirements.
Current statistics reveal that the drug sterility testing market is witnessing substantial growth, propelled by North America's dominance in drug innovation and a robust regulatory framework. This expansion necessitates comprehensive safety evaluations for new medications and biologics, emphasizing the critical demand for reliable separation techniques.
Furthermore, the Asia Pacific region is projected to exhibit the highest compound annual growth rate (CAGR) in this market from 2025 to 2030, indicating an increasing need for efficient purification solutions, with experts asserting that the use of a 0.2 um filter is crucial for maintaining sterility in drug manufacturing, as it adheres to the regulatory standards established for patient safety.
Regulatory specialists highlight that the application of these barriers is essential for deactivating microbial contaminants on non-sterile instruments, thereby safeguarding public health. As the industry progresses, reliance on the 0.2 um filter will continue to be a foundational element of drug manufacturing standards, ensuring that new treatments introduced to the market are both safe and effective.
Bacterial Filtration: How 0.2 µm Filters Enhance Product Integrity
The 0.2 um filter membranes are specifically engineered to capture bacteria, ensuring that only sterile solutions are utilized in medical applications. This purification method not only safeguards the product from contamination but also enhances its overall integrity. By hindering microbial growth, these devices play a crucial role in preserving the efficacy of active components, which is essential for the therapeutic effectiveness of medicinal products.
Recent studies indicate that microbial contamination remains a significant concern in drug manufacturing, with evidence suggesting that a notable percentage of items can be compromised by microbial presence. The application of the 0.2 um filter has been demonstrated to significantly reduce this risk, achieving bacterial retention rates exceeding 99.9%. Expert opinions underscore the importance of the 0.2 um filter; scientists in the drug industry have observed that its use not only protects the product but also enhances its stability and shelf life.
For instance, a case study involving pediatric patients receiving parenteral nutrition (PN) revealed that the use of a 0.2 um filter was linked to improved safety outcomes. This highlights the necessity for tailored filtration protocols in at-risk populations, particularly given the lack of specific guidelines on filtration for pediatric patients.
In summary, the incorporation of the 0.2 um filter membranes in pharmaceutical processes signifies a substantial advancement, ensuring that products meet stringent safety standards while maintaining their therapeutic effectiveness. Clinicians are encouraged to adhere to best practices for sterile solution administration, which involves utilizing these devices to safeguard patient health.
Sample Preparation: Utilizing 0.2 µm Filters for Accurate Analysis
In analytical laboratories, the use of a 0.2 um filter is crucial for effective sample preparation. These devices excel at eliminating particulate matter and microorganisms that can undermine analytical results. By ensuring samples are free from contaminants, the application of a 0.2 um filter significantly enhances the accuracy and reliability of various analytical techniques, including high-performance liquid chromatography (HPLC) and mass spectrometry.
Statistics indicate that contamination interference can lead to substantial inaccuracies in HPLC and mass spectrometry results, underscoring the necessity of precise filtration. For instance, cellulose acetate syringe membranes are especially efficient for purifying aqueous solutions with low protein binding, rendering them suitable for delicate applications.
Case studies demonstrate that utilizing a 0.2 um filter during sample preparation can lead to significant enhancements in analytical results. Laboratories that adopted these devices reported improved clarity and consistency in their results, which is essential for regulatory compliance and research integrity.
Expert insights from analytical chemists emphasize the pivotal role of filtration in HPLC and mass spectrometry, with many noting that the quality of filtration directly impacts the reliability of the data obtained. The consensus is clear: using a 0.2 um filter not only enhances analytical results but also simplifies the overall sample preparation procedure, making it an essential element in contemporary analytical laboratories.
Solvent Purification: The Importance of 0.2 µm Filters in Research
0.2 µm membranes play a pivotal role in solvent purification procedures, effectively eliminating impurities and particulates to ensure that solvents meet the highest quality standards. This is particularly critical in sensitive applications such as chromatography, where even minor impurities can jeopardize results.
A study on lipidomic profiling illustrates this point, revealing that the quality of organic solvents significantly affects the accuracy and sensitivity of lipid analysis in LC-MS. The research demonstrated that utilizing high-purity LC-MS-grade solvents, especially isopropanol, led to substantially improved signal intensity and enhanced detection of lipid species. Conversely, the use of lower-quality solvents resulted in drastically diminished intensities, with less than 15% of the original C fragment 603.5347 reaching the detector when the precursor ion was isolated with a 1 Da window.
This emphasizes the necessity for rigorous and routine monitoring of solvent quality. Researchers consistently depend on a 0.2 um filter for filtration to maintain the integrity of their experimental conditions, as it directly influences the reproducibility and reliability of their results. Expert opinions further underscore that stringent quality control measures in solvent purification are essential for achieving accurate outcomes in chromatography research.
Injectable Drug Production: Safeguarding Quality with 0.2 µm Filters
In the creation of injectable medications, utilizing a 0.2 um filter is paramount for ensuring product quality. These devices effectively eliminate bacteria and other impurities that could compromise the safety of injectable formulations. A significant statistic reveals that 78.6% of hospitals utilize lipidic devices for parenteral nutrition, underscoring the critical reliance on purification methods in clinical settings, which directly correlates with the implementation of the 0.2 um filter in the manufacturing of injectable drugs.
Implementing stringent screening processes not only safeguards patient health but also fulfills regulatory compliance standards. For instance, a comprehensive study involving 1,506 ICU patients demonstrated that effective purification is essential for achieving balanced treatment outcomes, thereby highlighting the necessity of employing 0.2 um filters in injectable formulations.
Pharmaceutical manufacturers recognize that improving the drug purification process is crucial for quality assurance. Expert opinions indicate that variations in neonatal infusion procedures may expose patients to risk, emphasizing the need for standardized filtering techniques. As one producer articulated, "The importance of purification in injectable formulations cannot be overstated; it is a fundamental step in ensuring patient safety."
The influence of filtration on the safety of injectable formulations in 2025 is increasingly apparent, as manufacturers endeavor to elevate product quality through rigorous filtration methods. By prioritizing the use of a 0.2 um filter, drug laboratories can significantly enhance the reliability and safety of their injectable products, ultimately benefiting patient care.
Regulatory Compliance: Meeting Standards with 0.2 µm Filtration
The implementation of 0.2 um filters is a fundamental requirement set by regulatory agencies to ensure the sterility and safety of medical products. Adhering to these stringent standards is essential for manufacturers; non-compliance can lead to significant penalties, including hefty fines and product recalls. Recent statistics indicate that companies encountering regulatory violations in drug manufacturing can incur penalties exceeding millions of dollars, underscoring the financial implications of failing to meet these critical standards.
Utilizing a 0.2 um filter not only mitigates contamination risks but also enhances product integrity, thereby reinforcing a company's commitment to quality and safety in its manufacturing processes. Case studies, including those related to the Octet platform, demonstrate how advanced purification technologies can enhance contamination detection capabilities, enabling accurate monitoring of potential contaminants such as host cell proteins and residual Protein A. This degree of precision is essential for upholding compliance and ensuring the safety of medicinal products.
Furthermore, expert views from compliance officers underscore the importance of meeting purification standards, emphasizing that strict adherence to the 0.2 um filter protocols is crucial for maintaining regulatory compliance and protecting public health. As the pharmaceutical landscape changes in 2025, the significance of these standards will only increase, making it essential for companies to invest in high-quality purification solutions to meet both current and future regulatory requirements.
To ensure compliance, lab managers should regularly assess their purification systems and consider upgrading to the latest technologies that incorporate a 0.2 um filter to meet or exceed standards.
Biopharmaceutical Applications: Leveraging 0.2 µm Filters for Biologics
In the biopharmaceutical industry, the use of 0.2 um filters is essential for biologics production, as they effectively eliminate contaminants that could compromise product safety and efficacy. Research indicates that contamination risks in biologics can significantly influence therapeutic outcomes, making the selection of appropriate purification techniques crucial. By employing a 0.2 um filter for purification, biopharmaceutical firms not only enhance product quality but also ensure compliance with stringent regulatory standards, which is vital for providing safer therapeutic options to patients.
Furthermore, the integration of Karl Fischer titration, particularly with the AQV-300 and AQ-300 models, plays a pivotal role in ensuring the accurate measurement of moisture content in pharmaceuticals. This accuracy is essential for maintaining the stability and efficacy of biologics. The combination of 0.2 um filter and precise titration techniques empowers companies to establish robust quality control measures, ultimately enhancing patient care.
For instance, a case study titled "Choosing the Right Partner for Purification" underscores the importance of selecting a reliable collaborator to meet specific processing requirements, including efficiency and chemical compatibility. Collaborating with knowledgeable representatives in the field can lead to effective contamination management and adherence to industry standards.
Moreover, expert perspectives emphasize that the use of a 0.2 um filter is critical in biopharmaceutical processes, particularly in sterile purification methods. These filters, with an average pore size of 0.26 µm, are vital for achieving the necessary level of sterility in biologics production. As highlighted by biopharmaceutical researchers, the impact of purification on product safety cannot be overstated, underscoring the necessity of implementing robust purification strategies to enhance the safety and efficacy of biologics. By prioritizing the 0.2 um filter alongside Karl Fischer titration, companies can significantly elevate the quality of their products, ultimately benefitting patient care.
Cost-Effectiveness: Maximizing Value with 0.2 µm Filters
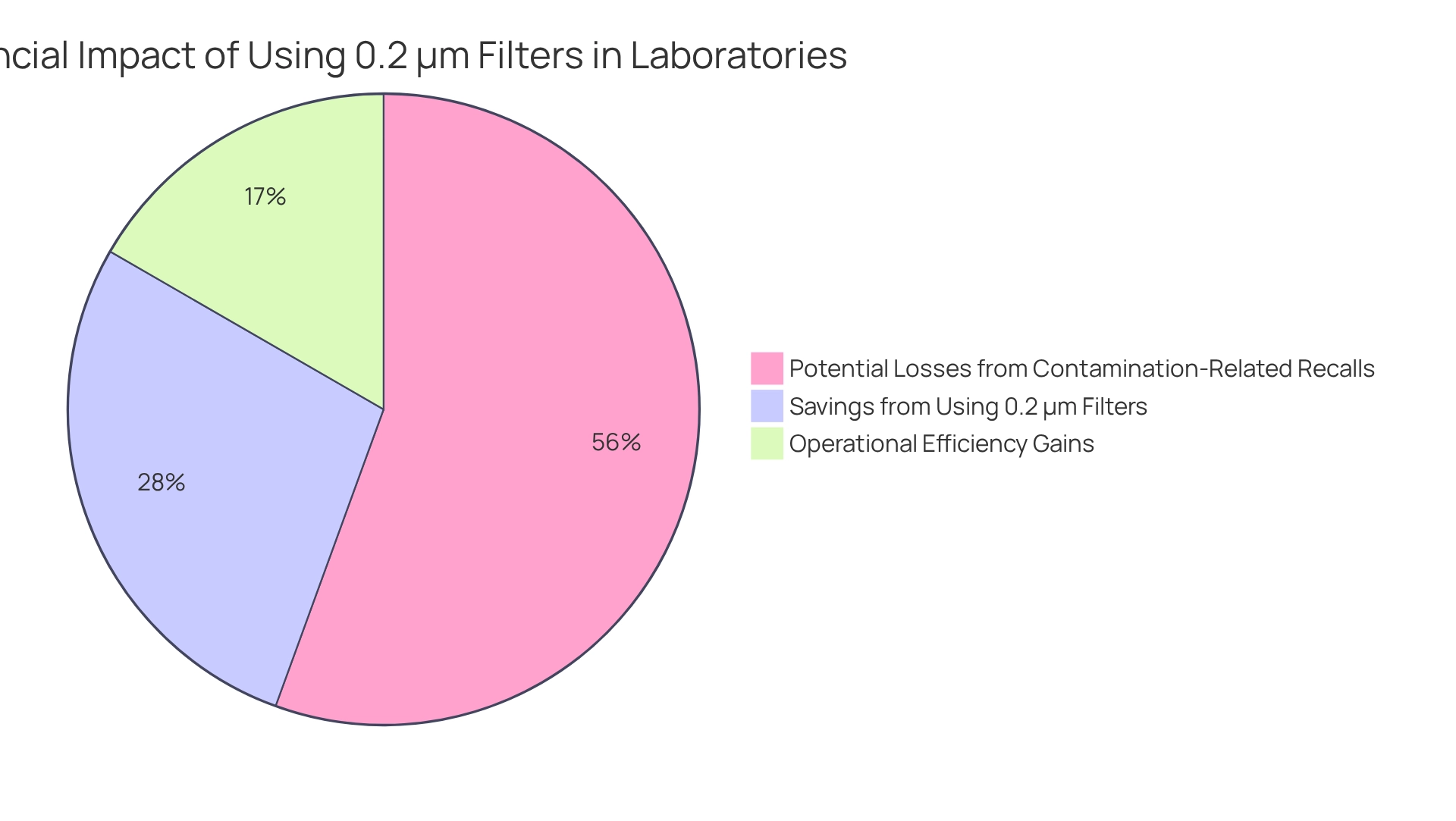
Utilizing a 0.2 um filter presents an exceptionally economical solution for laboratories and manufacturers. These filters are essential in preventing contamination, thereby safeguarding product integrity and significantly diminishing the risk of costly recalls and rework. Studies reveal that contamination-related recalls in pharmaceuticals can result in losses exceeding millions of dollars annually, with estimates indicating that the average recall cost can reach up to $10 million. By adopting a 0.2 um filter, laboratories can mitigate these risks, leading to substantial savings in both time and resources.
Moreover, the operational efficiency gained through the use of a 0.2 um filter constitutes a prudent investment for any laboratory or production facility. Financial analysts have observed that the economic advantages of implementing this filtration technology can bolster overall productivity and decrease operational costs. As one analyst noted, 'The use of a 0.2 um filter not only reduces contamination risks but also enhances resource allocation, leading to substantial cost savings over time.' A case study contrasting in-line devices with guard columns demonstrated that while in-line devices are favored for their cost-effectiveness, the long-term savings associated with 0.2 µm devices can exceed initial costs, particularly in critical environments such as drug manufacturing.
In conclusion, the strategic application of the 0.2 um filter not only elevates product quality but also delivers significant financial advantages, establishing it as a vital component in modern drug manufacturing laboratories.
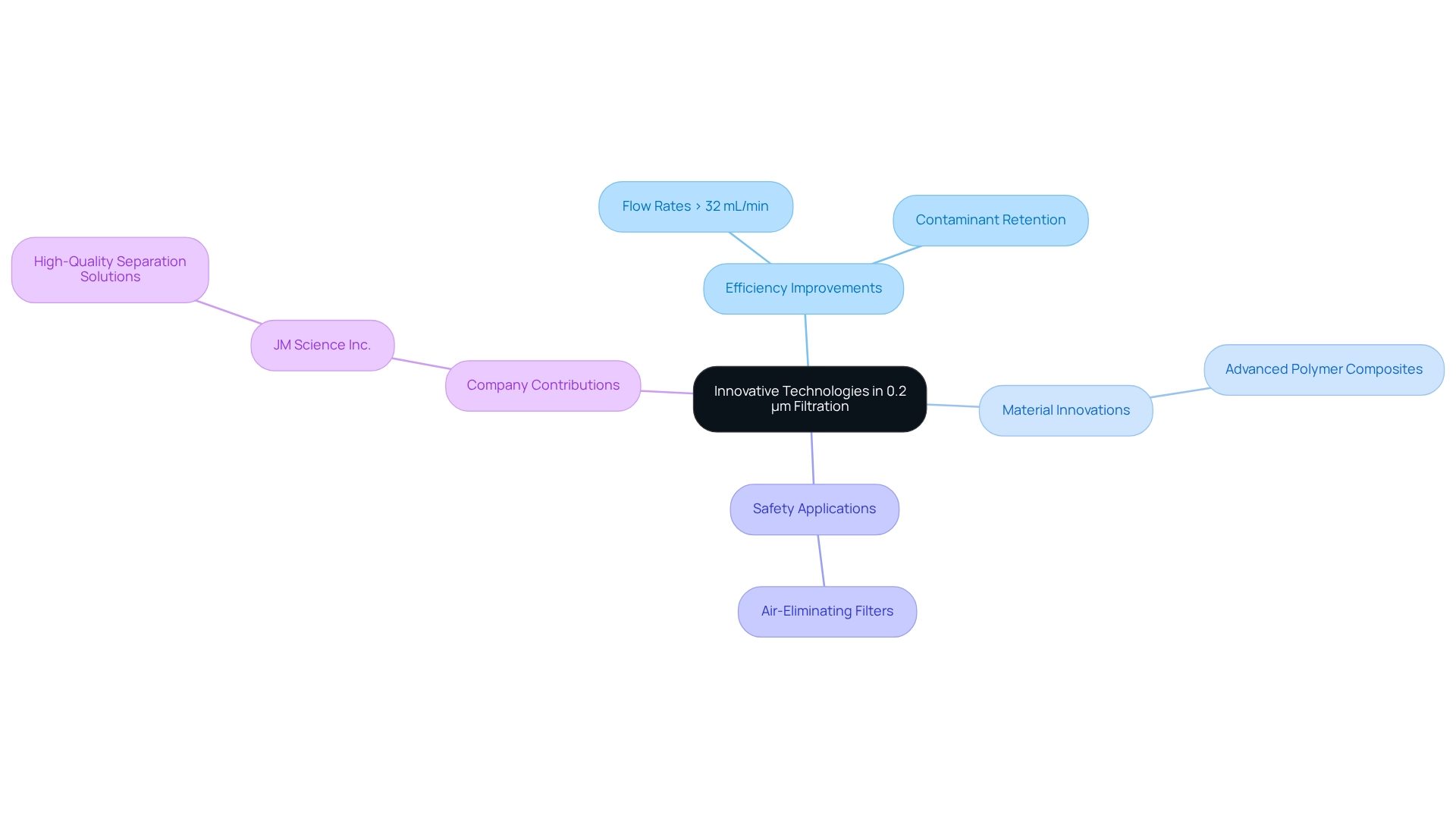
Innovative Technologies: Advancements in 0.2 µm Filtration Solutions
Recent advancements in filtration technologies utilizing a 0.2 um filter have significantly enhanced the efficiency and reliability of filtration processes in laboratories. Innovations in membrane materials, particularly the introduction of advanced polymer composites, have led to devices that not only achieve quicker flow rates—exceeding 32 mL/min at 80 cm water head pressure—but also exhibit improved contaminant retention capabilities. These enhancements are essential for ensuring the purity and safety of pharmaceutical products, as they effectively eliminate particulates and microorganisms that could compromise product integrity.
Furthermore, the integration of air-eliminating filters into standard clinical practices is recommended to prevent vascular air embolism, illustrating the practical applications of these technologies in enhancing patient safety. JM Science Inc. plays a critical role in these advancements by providing high-quality separation solutions that align with the latest innovations in the industry. As Alan Kay wisely stated, "The best method to foresee the future is to create it," and JM Science is committed to shaping the future of purification technologies. As the industry evolves, the latest innovations in 0.2 um filter solutions are establishing new standards for quality assurance in pharmaceutical manufacturing, ultimately contributing to improved health outcomes and enhanced research capabilities.
Conclusion
The significance of 0.2 µm filters in pharmaceutical manufacturing is paramount. These filters are essential for ensuring sterility, enhancing product integrity, and maintaining compliance with stringent regulatory standards. By effectively removing bacteria and other contaminants, they safeguard critical processes such as injectable drug production, sample preparation, and solvent purification. Advancements in filtration technology have further solidified their role, demonstrating impressive filtration efficiency and reliability.
As the pharmaceutical landscape evolves, the reliance on 0.2 µm filters is set to increase, driven by growing regulatory demands and the need for uncompromised product safety. The financial implications of adhering to these standards are substantial; the cost-effectiveness of implementing these filters showcases their value in preventing contamination-related losses. With a projected growth in market share, these filters are becoming indispensable tools for laboratory managers and pharmaceutical manufacturers dedicated to quality assurance.
Investing in 0.2 µm filtration technology transcends mere regulatory compliance; it is a strategic move towards ensuring patient safety and enhancing the overall efficacy of pharmaceutical products. As the industry continues to innovate, the commitment to high-quality filtration solutions will play a pivotal role in shaping the future of pharmaceutical manufacturing and research, ultimately benefiting public health and safety.




