Overview
This article delves into the essential facts surrounding pH electrodes, underscoring their critical role in laboratory environments for precise pH measurements. It elaborates on their construction, various types, calibration methods, and best storage practices, as well as their significance across diverse scientific and industrial applications. Reliable pH electrodes are indispensable for preserving the integrity of experimental data and ensuring adherence to industry standards. By understanding these components, laboratory professionals can enhance their operational accuracy and compliance.
Introduction
Understanding the intricacies of pH measurement is essential for laboratory managers committed to achieving precision in their analyses. pH electrodes are fundamental to obtaining accurate readings across various scientific disciplines, influencing everything from chemical experiments to medical diagnostics. As technology surrounding these sensors advances, it becomes increasingly vital for lab managers to remain informed about the critical aspects of their functionality, calibration, and maintenance.
What challenges may arise in ensuring the reliability of these indispensable instruments? How can managers adeptly navigate these challenges to uphold the integrity of their laboratory results?
JM Science pH Electrodes: High-Quality Solutions for Accurate Measurements
JM Science presents an extensive array of high-quality pH electrodes, meticulously engineered for precision and reliability across . Accurate pH measurements using pH electrodes are essential in disciplines such as chemistry, biology, and medical diagnostics, where even slight deviations can profoundly affect outcomes. These conductive elements are constructed from advanced materials and cutting-edge technologies, guaranteeing exceptional durability and consistent performance. As a result, they have become a trusted choice for managers and researchers prioritizing precision and dependability in their analytical processes.
Looking ahead to 2025, the pH sensor market is expected to evolve, with leading brands focusing on innovation and quality. The latest advancements in pH electrodes technology showcase enhancements in sensing capabilities and improved connectivity options, facilitating seamless integration into modern research environments. These innovations are vital for preserving the integrity of experimental data and ensuring adherence to industry standards, such as those specified in CFR 21 Part 11. Superior components are crucial for laboratories striving to maintain stringent quality control measures and comply with regulatory requirements.
How pH Electrodes Function: The Science Behind Accurate pH Measurement
pH electrodes play a crucial role in laboratory analyses by assessing the electrical potential difference between hydrogen ions in a solution and a reference sensor. The glass membrane of these sensors incorporates pH electrodes that are specifically designed to be sensitive to H+ ions, allowing for precise detection of variations in acidity or alkalinity. This potential difference is subsequently converted into using pH electrodes, which provide accurate readings essential for various scientific applications. Understanding the functionality of pH sensors is vital for ensuring the reliability of experimental results, reinforcing their importance in any laboratory setting.
Key Components of pH Electrodes: Understanding Their Structure and Functionality
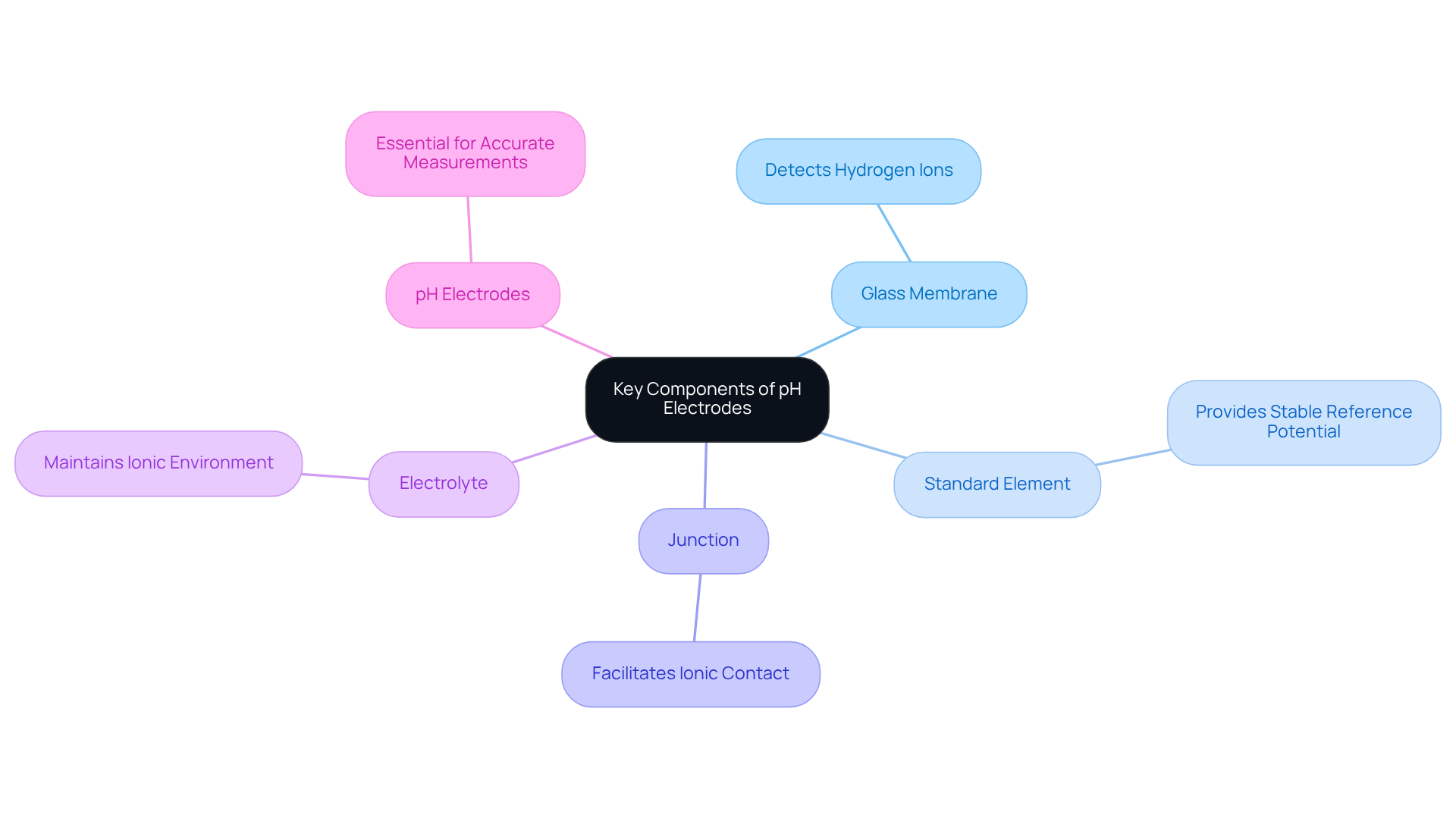
A typical pH sensor consists of several essential components, including:
- The glass membrane
- The standard element
- The junction
- The electrolyte
- The pH electrodes
The glass membrane plays a critical role in detecting hydrogen ion concentration, which is fundamental for accurate measurements. Meanwhile, the reference component provides a stable reference potential, ensuring reliability in readings. The junction facilitates ionic contact between the conductor and the solution, while the electrolyte maintains the necessary ionic environment for precision. Understanding these components is vital for appreciating the significance of , including pH electrodes, in laboratory settings.
Types of pH Electrodes: Choosing the Right One for Your Application
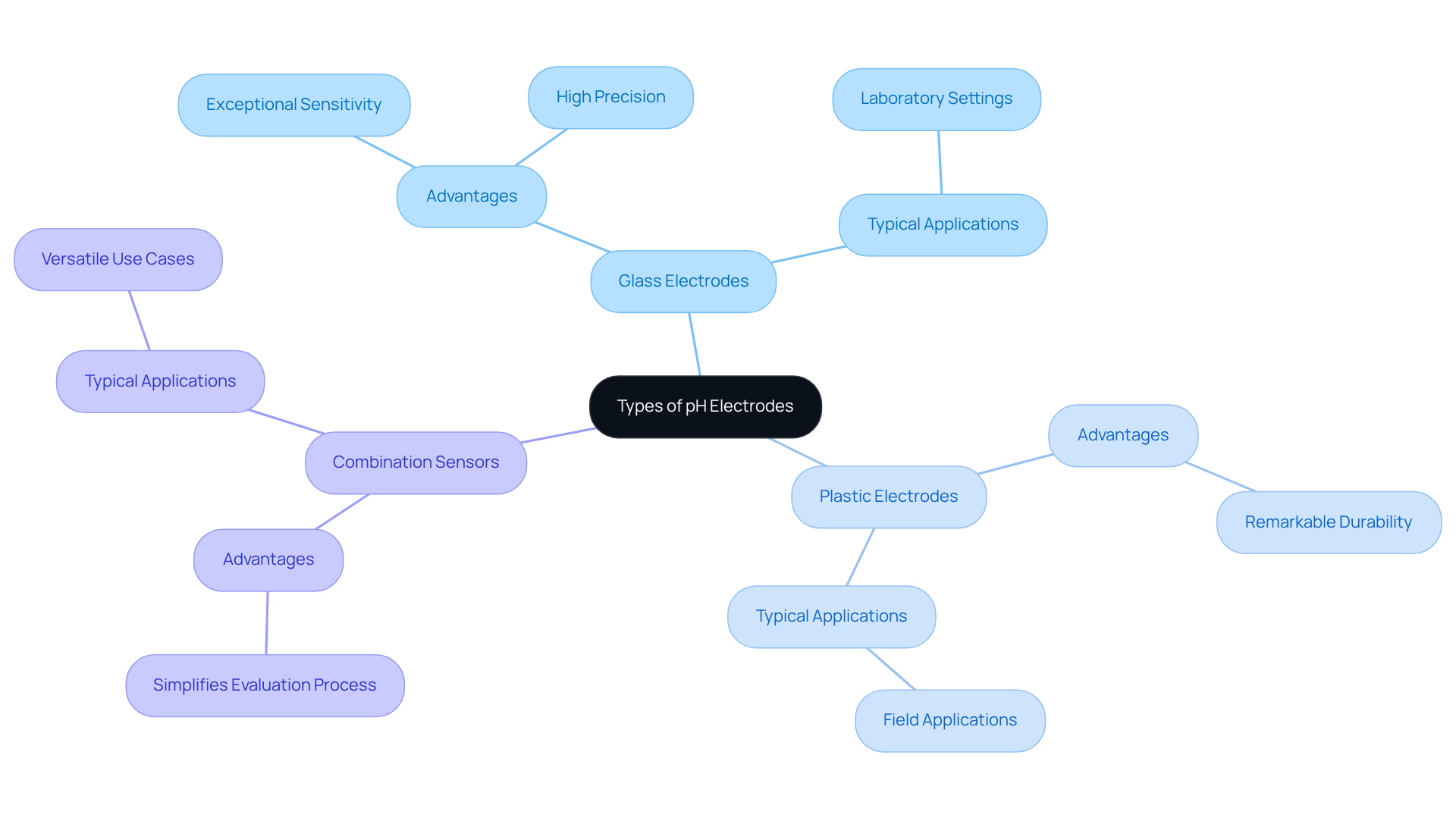
In the realm of pH electrodes, various types are available, such as glass, plastic, and combination sensors. pH electrodes, known for their exceptional sensitivity and precision, contribute to the dominance of glass sensors in the market, making them a preferred choice in many laboratory settings. On the other hand, plastic sensors are frequently employed in field applications, thanks to their remarkable durability. Combination sensors, which integrate both sensing and reference components into a single unit, simplify the evaluation process significantly.
Selecting the appropriate type hinges on the specific requirements of the application, such as the temperature range and sample composition, underscoring the importance of in achieving accurate results.
Calibration of pH Electrodes: Ensuring Accurate and Reliable Measurements
To ensure precise measurements, it is imperative that pH electrodes undergo regular calibration using . A two-point calibration with pH electrodes is highly recommended, typically involving pH 4 and pH 7 buffers. This critical process adjusts the response of the pH electrodes to align with established pH values, effectively compensating for any drift or performance variations that may occur over time. Regular calibration is essential not only for the accuracy of measurements but also for maintaining the integrity of laboratory results, reinforcing the reliability of scientific findings.
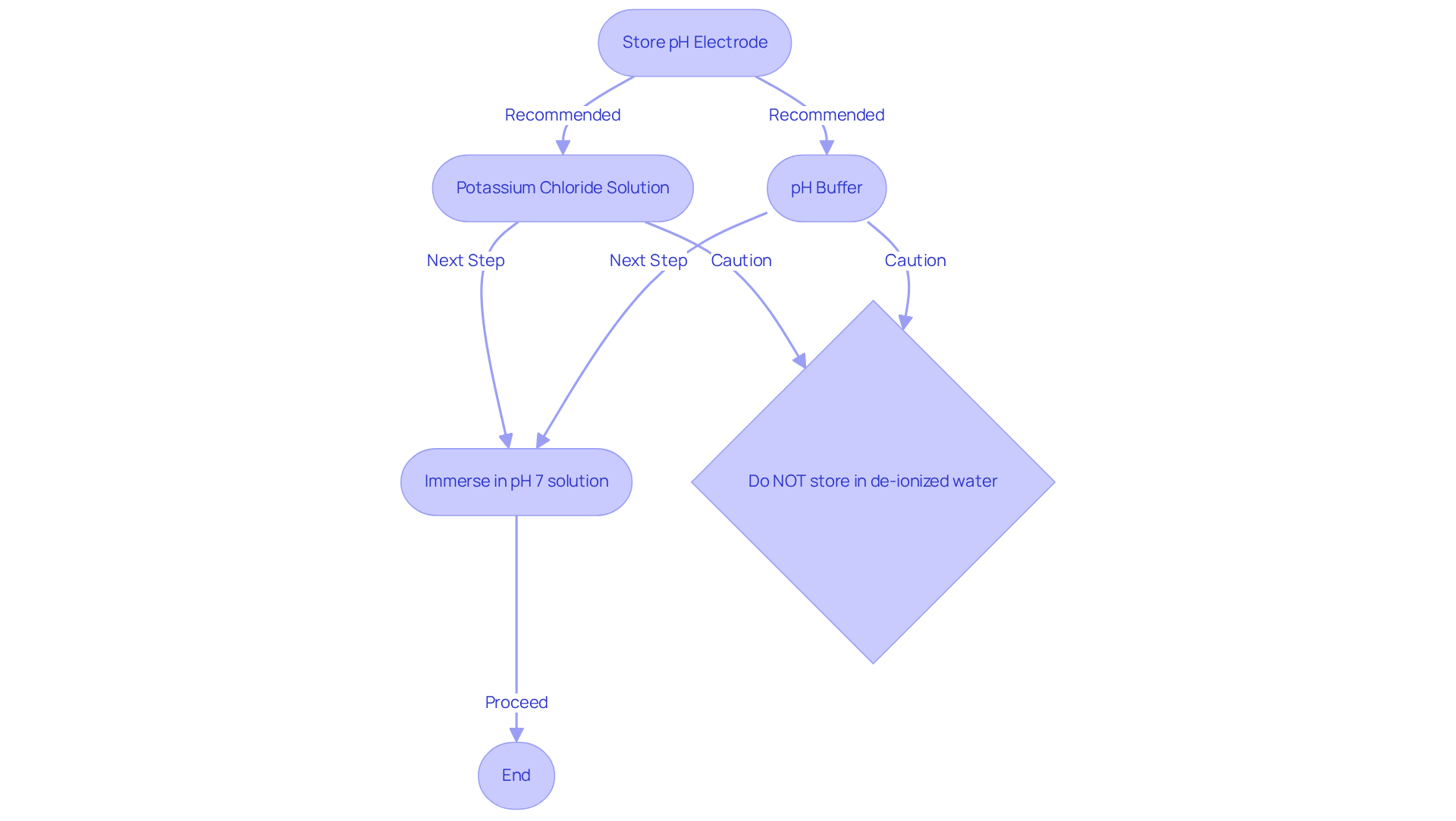
Proper Storage of pH Electrodes: Best Practices for Longevity
To ensure the longevity of pH electrodes, it is essential to , such as a potassium chloride (KCl) solution or a pH buffer. Keeping sensors dry can severely harm the delicate glass membrane, resulting in erroneous readings and potential replacement expenses. It is important to note that storing the probe in de-ionized water is inadvisable, as it depletes ions from the component, reducing its lifespan.
Between assessments, immersing sensors in a pH 7 solution or pure water is recommended to preserve moisture and optimal functionality. However, while placing the probe in a storage buffer may revive dried sensors, it can jeopardize the accuracy of the readings. Maintaining a high ion concentration around the terminals is crucial for pH electrodes; therefore, using a storage buffer made from one part potassium chloride mixed with 99 parts deionized water is advisable.
This method not only preserves the integrity of the glass membrane but also enhances the sensitivity of the sensors, ensuring precise pH readings across various applications. Adequate hydration is vital, as dried sensors can lead to malfunction and reduced measurement precision. After rinsing, the pH probe should be blotted dry with soft tissue to prevent contamination.
These consistent and correct storage methods are essential in laboratory settings.
Common Issues with pH Electrodes: Troubleshooting Tips for Lab Managers
Common issues with pH electrodes can significantly impact laboratory results, such as erratic readings, slow response times, and drift in calibration. To effectively troubleshoot these problems, it is essential to ensure that the probe is clean and properly hydrated. If readings remain unstable, inspect the glass membrane for cracks or check for impurities on .
Regular maintenance and cleaning are crucial; they can prevent many of these issues and ensure the reliable performance of your instruments. By addressing these concerns proactively, you can maintain the integrity of your measurements and uphold the quality of your scientific work.
The Role of Reference Electrodes in pH Measurement: A Critical Component
Reference sensors are essential for , providing a stable potential against which the pH of a solution is assessed. Their primary function is to ensure that any variations in the measured potential reflect changes in the sample's pH rather than fluctuations in the sensor's performance. To achieve [accurate and reliable pH measurements](https://jmscience.com), it is crucial to maintain a standard device properly, as the potential difference should ideally remain below 2-3 mV to be considered stable. If the potential difference exceeds 5 mV, the measuring device must be replaced or disposed of. Research indicates that various factors, such as temperature and contamination, can affect the stability of reference devices, underscoring the importance of regular inspection and calibration. Calibration with buffer solutions of known pH is necessary at least monthly to ensure precise pH measurements.
In practical laboratory environments, maintaining reference devices involves routine cleaning and appropriate storage. For instance, storing sensors in potassium chloride or diluted sulfuric acid solutions prevents them from drying out, which can compromise their performance. Additionally, employing self-cleaning systems or ultrasonic cleaning methods—such as an ultrasonic generator operating at 25 kHz connected to the assembly—can significantly reduce maintenance frequency, enhancing the reliability of pH readings in continuous monitoring applications.
Real-world examples highlight the importance of benchmark terminals across various scientific fields. In clinical diagnostics, for example, accurate blood pH measurement—typically maintained around 7.40—is crucial for patient care. As Yitzhak Mendelson noted, "The assessment of blood pH is fundamental to many diagnostic procedures." The use of consistent benchmark devices ensures that healthcare professionals can rely on accurate measurements, which are vital for diagnosing and managing medical conditions. Ultimately, the role of reference sensors in pH assessment is indispensable, as they are critical to ensuring the precision and reliability of laboratory analyses.
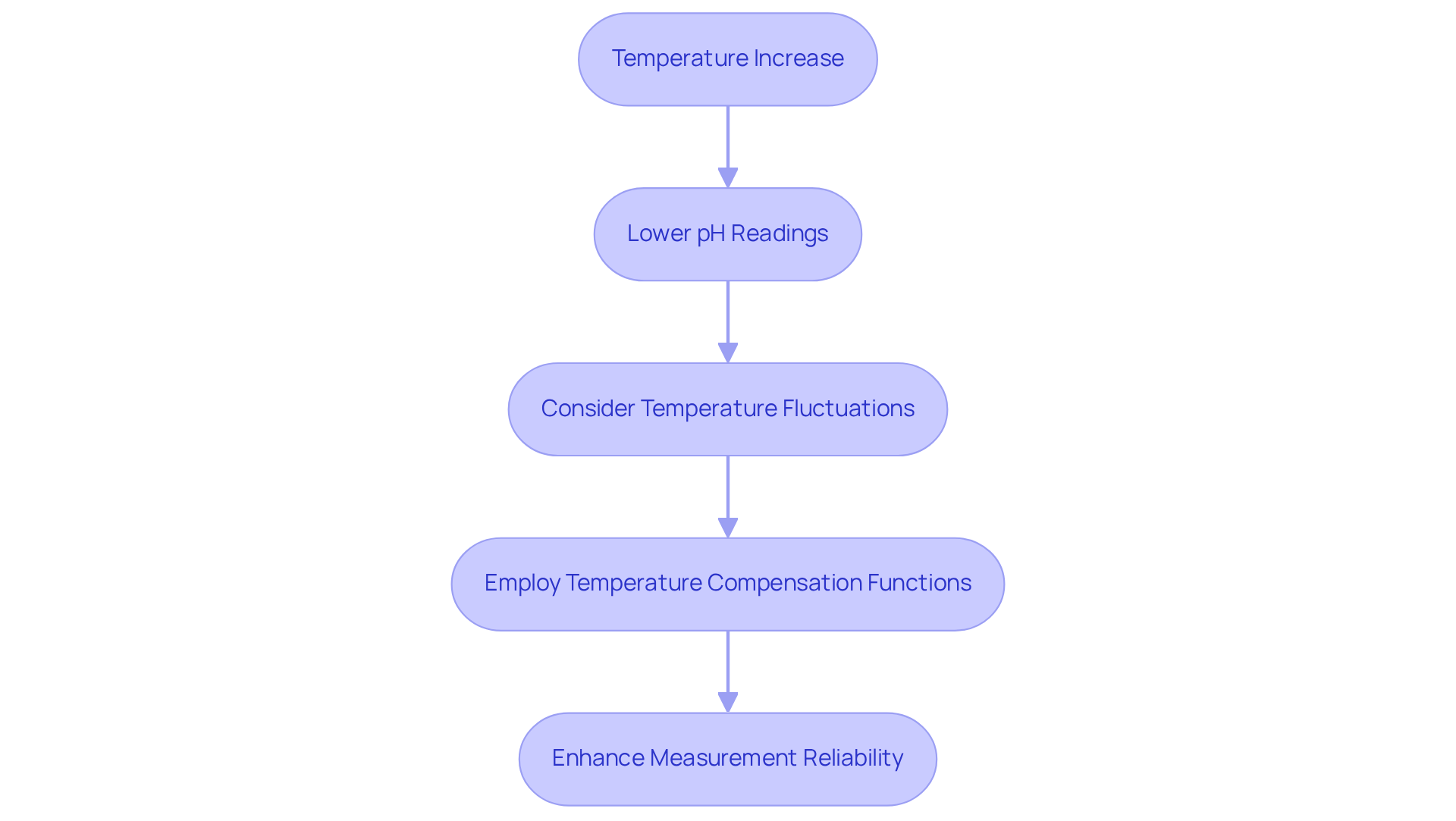
Temperature Effects on pH Measurements: Understanding the Influence
Temperature significantly influences the pH of a solution; higher temperatures typically result in lower pH readings. This phenomenon occurs due to increased ion mobility and alterations in the dissociation of water. Consequently, lab supervisors must account for temperature fluctuations during assessments. They should also consider employing temperature compensation functions in pH electrodes to ensure accurate results across varying conditions. By prioritizing these factors, laboratories can enhance the reliability of their measurements, ultimately leading to more precise scientific outcomes.
The Importance of pH Electrodes in Scientific Research and Industry Applications
pH sensors serve as indispensable tools in scientific research and a range of industries, such as pharmaceuticals, food and beverage, and environmental monitoring. Accurate pH measurements are not merely beneficial; they are crucial for quality control, product formulation, and adherence to regulatory standards. The reliability of pH electrodes plays a significant role in the success of experiments and processes, underscoring their essential importance in . Understanding the impact of these sensors on operational outcomes underscores the need for high-quality scientific instruments. Consequently, investing in reliable pH electrodes is not only a strategic decision but also a necessary step toward ensuring success in various applications.
Conclusion
Understanding the intricacies of pH electrodes is crucial for laboratory managers who aim to ensure precision in their analytical processes. These essential instruments not only provide accurate pH measurements but also uphold the integrity of experimental results across various scientific disciplines. As technology continues to advance, the importance of selecting high-quality pH electrodes, maintaining their functionality, and adhering to best practices in calibration and storage cannot be overstated.
This article delves into key aspects of pH electrodes, including their construction, functionality, types, and the significance of proper calibration and storage. Insights into common issues and troubleshooting tips further highlight the need for regular maintenance to ensure reliable performance. Additionally, understanding the role of reference electrodes and the impact of temperature on pH measurements reinforces the complexity involved in achieving accurate results.
Ultimately, investing in high-quality pH electrodes is not merely a technical decision; it is a strategic imperative for laboratory managers committed to excellence in scientific research and industry applications. By prioritizing the reliability and accuracy of these instruments, laboratories can enhance their operational outcomes and maintain compliance with regulatory standards, paving the way for successful experiments and processes.




