Overview
A pH buffer serves as a crucial chemical solution that maintains stable pH levels, even when acids or bases are introduced. Typically composed of a weak acid paired with its conjugate base or a weak base with its conjugate acid, these buffers play an essential role in various scientific applications. The significance of pH buffers is particularly evident in pharmaceutical formulations and biochemical assays, where they stabilize pH. This stabilization ensures the reliability and accuracy of experimental results, ultimately impacting drug efficacy. Understanding the function of pH buffers is vital for anyone engaged in scientific research, as their role cannot be overstated.
Introduction
In the realm of scientific research and laboratory practices, pH buffers emerge as critical components, ensuring the stability and reliability of countless experiments. These specialized solutions are engineered to resist fluctuations in pH, playing an indispensable role across various applications, from drug formulation in pharmaceutical laboratories to enzyme activity in biochemical assays.
As the demand for precision and accuracy in scientific endeavors escalates, a comprehensive understanding of the mechanisms and types of pH buffers becomes essential for professionals in the field. With innovations on the horizon and a growing emphasis on sustainability, the evolution of pH buffer technology is poised to enhance laboratory efficiency and reliability. This makes the study of pH buffers not only relevant but imperative for the future of scientific exploration.
Defining pH Buffers: A Fundamental Overview
A pH buffer is a specific mixture designed to withstand notable fluctuations in pH when minor quantities of a sour or alkaline substance are added. Typically composed of a weak acid paired with its conjugate base or a weak base with its conjugate counterpart, this equilibrium allows the system to neutralize added substances, thus preserving a stable pH. For instance, a commonly utilized solution made of acetic acid and sodium acetate effectively stabilizes the pH around 4.75.
The significance of pH buffer stabilizers in laboratory environments cannot be overstated. They are essential for calibrating systems that utilize pH buffers, ensuring accurate measurements crucial for quality control, method validation, and instrument qualification. Notably, the CO2-bicarbonate system can manage an impressive 15,000-20,000 mmol of acid per day, showcasing the buffering capacity vital for maintaining physiological pH levels.
However, it is important to note that bone neutralization is a slow process due to limited extracellular fluid flow and the necessity for calcium elimination, adding complexity to the neutralization processes in biological systems.
Expert opinions underscore that pH buffers play a pivotal role in various chemical processes. As highlighted in a technical paper on pH buffer solutions, are characterized by traceability, stability, and certification, which are critical for reliable laboratory results. The document also offers practical advice on selecting suitable pH buffer solutions, noting the development of available choices, with Reagecon recognized for its extensive variety of high-quality pH buffer options.
Furthermore, the term 'open reservoir' is referenced in the literature, as noted by McNamara and Worthley, indicating its specific relevance in physiological contexts.
Real-world applications of pH solutions are abundant in chemistry. For example, they are employed in biochemical assays, where maintaining a specific pH buffer is crucial for enzyme activity and reaction rates. Additionally, in pharmaceutical facilities, the use of pH buffers is essential for preparing medication mixtures, ensuring that the active components remain stable and effective.
pH buffer standard solutions that encompass the complete pH range are utilized for calibrating pH measuring systems, further emphasizing their importance in scientific environments.
In conclusion, understanding the definition and significance of pH solutions is essential for professionals in chemistry and related fields. These mixtures support numerous scientific processes and reactions, enabling precise and dependable scientific work.
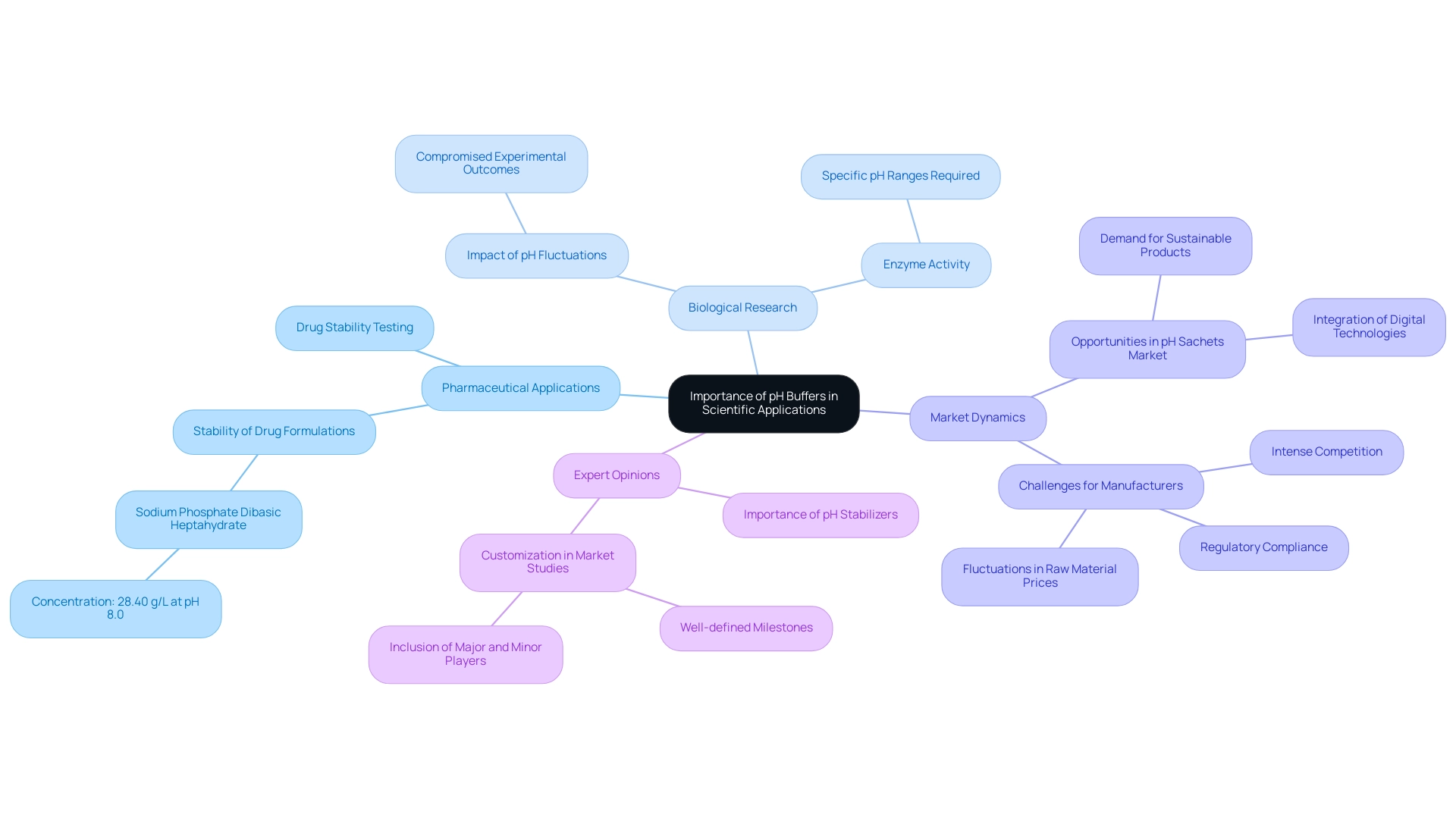
The Importance of pH Buffers in Scientific Applications
pH buffers are indispensable in various scientific applications, particularly within pharmaceutical laboratories, where they are vital for preserving the stability of drug formulations. By maintaining stable pH buffer levels during both production and storage, these agents ensure the efficacy of medications is upheld. For instance, Sodium Phosphate Dibasic Heptahydrate is utilized at a concentration of 28.40 g/L at pH 8.0, showcasing its effectiveness in sustaining optimal conditions for drug stability.
In biological research, the significance of solutions extends to enzyme activity, as many enzymes necessitate specific pH ranges to function effectively. The absence of stabilizers can lead to minor fluctuations in the pH buffer, potentially compromising experimental outcomes or resulting in unsuccessful experiments. This underscores the essential role of pH buffers in scientific research and development.
Recent studies have delved into the opportunities and challenges present in the pH sachets market, highlighting a rising demand for sustainable products alongside the integration of digital technologies. However, manufacturers encounter obstacles such as intense competition and regulatory compliance, which can affect the availability and efficacy of pH solutions in laboratories. As discussed in the case study titled "Opportunities and Challenges in the pH Sachets Market," these dynamics are crucial for understanding the current landscape of pH applications.
Expert opinions emphasize the importance of pH stabilizers and the function of pH buffers in drug formulation. Many professionals acknowledge that maintaining pH stability is critical for the effectiveness of pharmaceutical products. For example, during drug stability testing, pH buffer solutions are routinely employed to ensure formulations remain within their optimal pH range, thereby enhancing their reliability and performance. The Marketing Director noted, "The study was customized to our targets and needs with well-defined milestones. We were impressed by the in-depth customization and inclusion of not only major but also minor players across the globe."
Overall, the function of pH buffer stabilizers in pharmaceutical facilities and biological research is paramount. Their ability to stabilize pH levels directly impacts and the precision of scientific experiments, establishing them as a cornerstone of contemporary practices. Furthermore, understanding the detailed market analysis for each country, including healthcare expenditure and regulatory influences, is essential for appreciating the broader significance of pH stabilizers within the pharmaceutical industry.
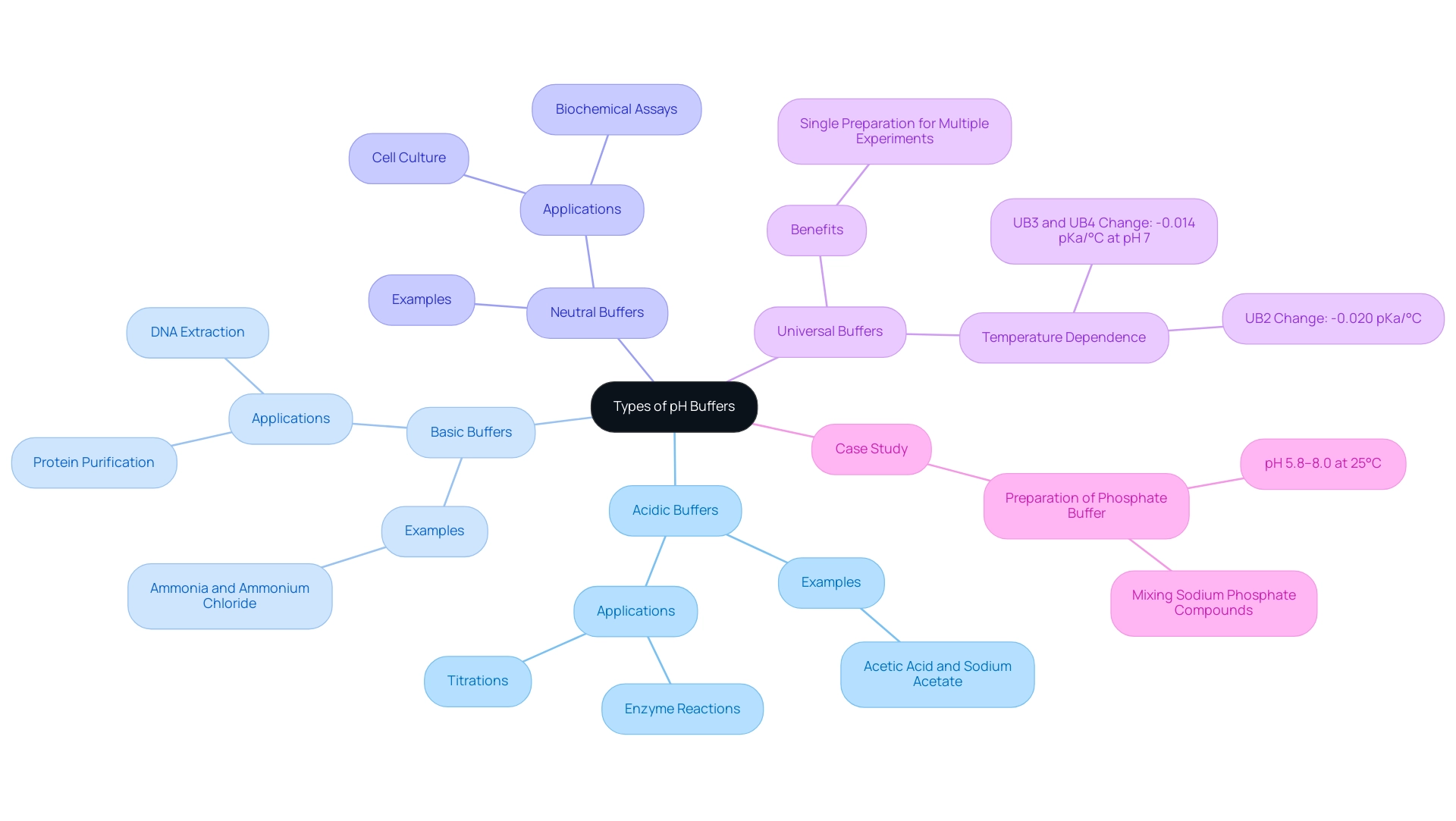
Exploring Different Types of pH Buffers: Acidic, Basic, and Neutral
pH buffers are essential tools in laboratory settings, categorized into three primary types: acidic, basic, and neutral. Acidic solutions, such as those composed of acetic acid and sodium acetate, are designed to maintain a pH below 7, making them ideal for reactions that necessitate a lower pH environment. Basic solutions, including ammonia and ammonium chloride, stabilize pH levels above 7, which is crucial for various biochemical processes.
Neutral solutions, typically around pH 7, are often employed in biological applications to mimic physiological conditions, ensuring experiments accurately represent the natural environment of biological systems.
Understanding the specific uses of these solutions is crucial for effective experimental work. Acidic solutions are commonly utilized in titrations and enzyme reactions, where a controlled acidic environment is necessary. For instance, titrations transitioning from high to low pH can be conducted through the stepwise addition of 5M hydrochloric acid, illustrating the practical application of acidic solutions in laboratory settings.
Basic solutions, , find applications in processes such as protein purification and DNA extraction, where maintaining a higher pH is essential for optimal results. Neutral agents are frequently employed in cell culture and biochemical assays, where sustaining physiological pH is vital for cell viability and function.
Recent studies underscore the significance of universal agents in biochemical research, enabling a single preparation to be used across multiple experiments. This innovation simplifies experimental design and enhances reproducibility. V.S. Stoll emphasizes this in his work, stating, "...
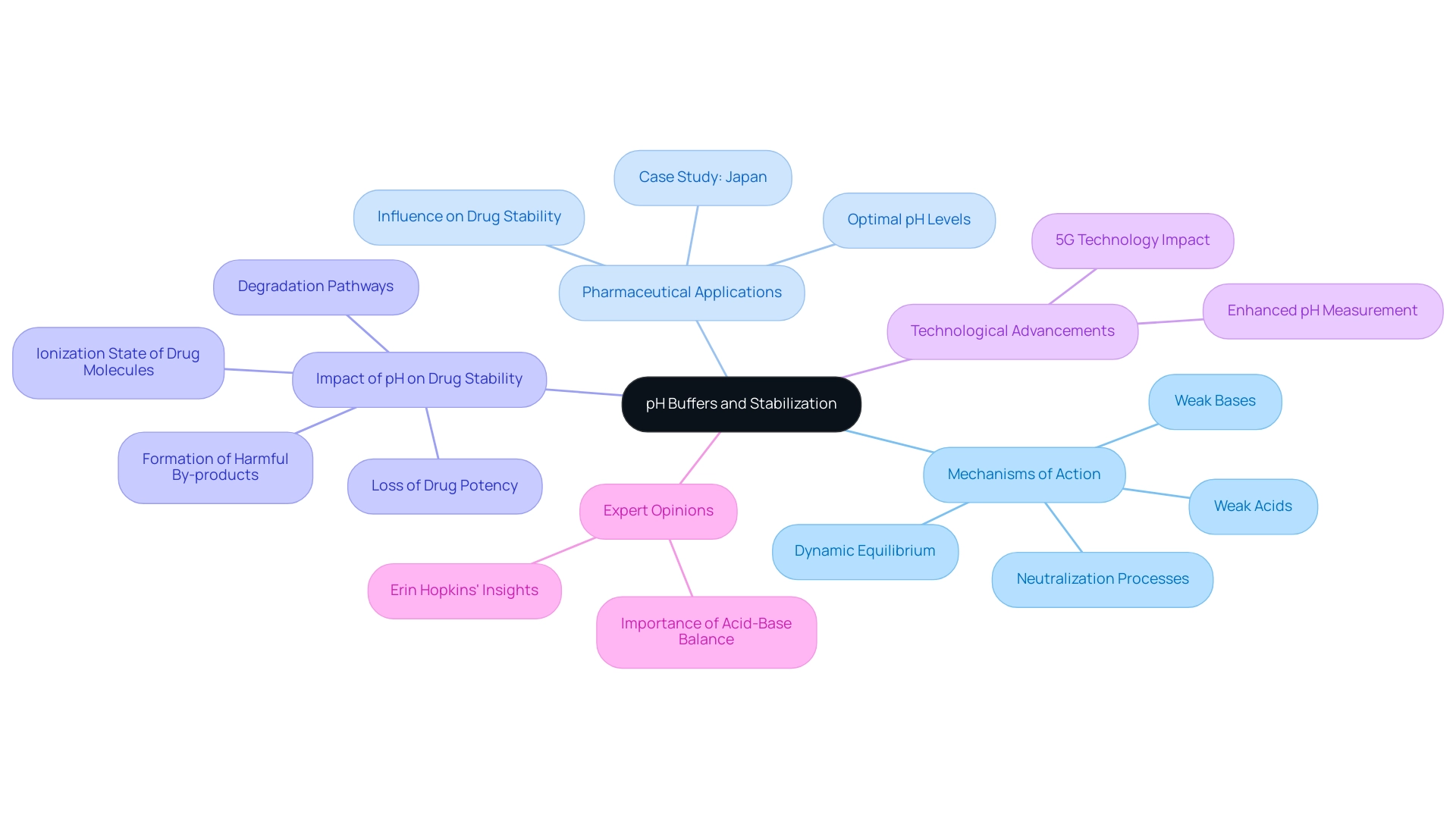
How pH Buffers Work: Mechanisms of pH Stabilization
pH buffers function through a dynamic equilibrium involving a weak acid and its conjugate base (or a weak base and its conjugate acid). This equilibrium is essential for maintaining across various chemical environments. When an acidic substance is introduced to the neutralizing mixture, the weak base interacts with the excess hydrogen ions (H+) to form a weak sour compound, effectively mitigating any change in pH.
Conversely, when a base is added, the weak component donates protons (H+) to neutralize the hydroxide ions (OH-), thereby stabilizing the pH once again. This dual capacity to neutralize small quantities of added acid or base is what renders these solutions indispensable as pH buffers in experimental environments.
The practical applications of these solutions underscore their critical role in pharmaceutical formulations, particularly concerning pH buffering. A notable case study conducted in Japan investigated the influence of pH on the stability of pharmaceutical compounds. The research highlighted how pH impacts the ionization state of drug molecules, potentially leading to various degradation pathways.
Findings revealed that extreme pH levels could catalyze hydrolysis and oxidation, culminating in a loss of drug potency and the formation of harmful by-products. This underscores the necessity of maintaining optimal pH buffer levels in pharmaceutical formulations to ensure drug stability, efficacy, and safety, thereby promoting rigorous pH control measures within the industry.
Moreover, advancements in technology, such as the rollout of 5G in Japan resulting in a 35% increase in mobile internet usage, illustrate the importance of contemporary technological progress in research settings. Such advancements can enhance the precision and efficiency of pH measurement and control, further emphasizing the relevance of pH buffers and stabilizers in modern pharmaceutical practices.
Expert opinions further reinforce the significance of pH stabilizers. Chemists emphasize that the ability of solutions to neutralize acids and bases is vital for maintaining pH buffers in both biological systems and experimental contexts. As Erin Hopkins articulates, 'Acid-base balance in the human body is one of the most paramount physiological processes.'
This highlights the broader implications of pH stabilization, extending beyond experimental environments into critical physiological functions.
In conclusion, the processes of pH stabilization, particularly through the utilization of pH buffers in solutions, are fundamental for ensuring consistent and reliable outcomes in scientific inquiry and applications. Their efficacy in neutralizing acids and bases not only supports scientific practices but also plays a pivotal role in the pharmaceutical industry, where maintaining drug stability is essential for patient safety and therapeutic efficacy. Furthermore, ongoing advancements in the field, including systematic guidelines for engineering active surfaces of charged peptides, further underscore the evolving characteristics of stabilizing mixtures and their applications in enhancing separation and filtration performance.
Practical Applications of pH Buffers in Laboratories
In research settings, pH buffers play an essential role across various applications, including titrations, spectrophotometry, and cell culture. During titrations, stabilizers are vital for maintaining a stable pH buffer, which is critical for accurately determining the concentration of unknown solutions. This stability facilitates high analytical throughput, as evidenced by studies employing multicommutated flow titration procedures that achieve remarkable accuracy in measuring total acidity in silage extracts.
As Carlo Maccà noted, 'Accuracy and precision are very good,' underscoring the importance of pH buffer solutions in attaining these crucial metrics in laboratory analyses.
In spectrophotometric analyses, the constancy of the pH buffer provided by stabilizing solutions is indispensable for ensuring reliable absorbance measurements. Fluctuations in pH can lead to significant variations in results, compromising the integrity of the analysis. The use of stabilizers in this context not only enhances precision but also aligns with the expectations of analytical suitability, as demonstrated by pH buffer chelatometric titrations that maintain a constant pH during the titration process, achieving commendable accuracy and efficiency compared to traditional methods.
Furthermore, in cell culture applications, pH buffer solutions are crucial for sustaining the physiological pH necessary for optimal cell growth and function. This is particularly significant in biological research, where even minor deviations in pH can adversely impact cellular processes. The consistent application of intermediaries in these settings emphasizes their essential role in promoting reliable experimental outcomes.
Overall, the integration of pH buffer stabilizers in laboratory practices not only enhances the accuracy and reliability of various analytical techniques but also supports the foundational requirements of biological research, establishing pH buffer as an indispensable component in the toolkit of any laboratory professional. Additionally, the amendment rate of , quantified at 80 t ha(-1)y(-1) for three years, illustrates the quantitative impact of pH stabilizers in environmental analyses, further highlighting their versatility and significance.
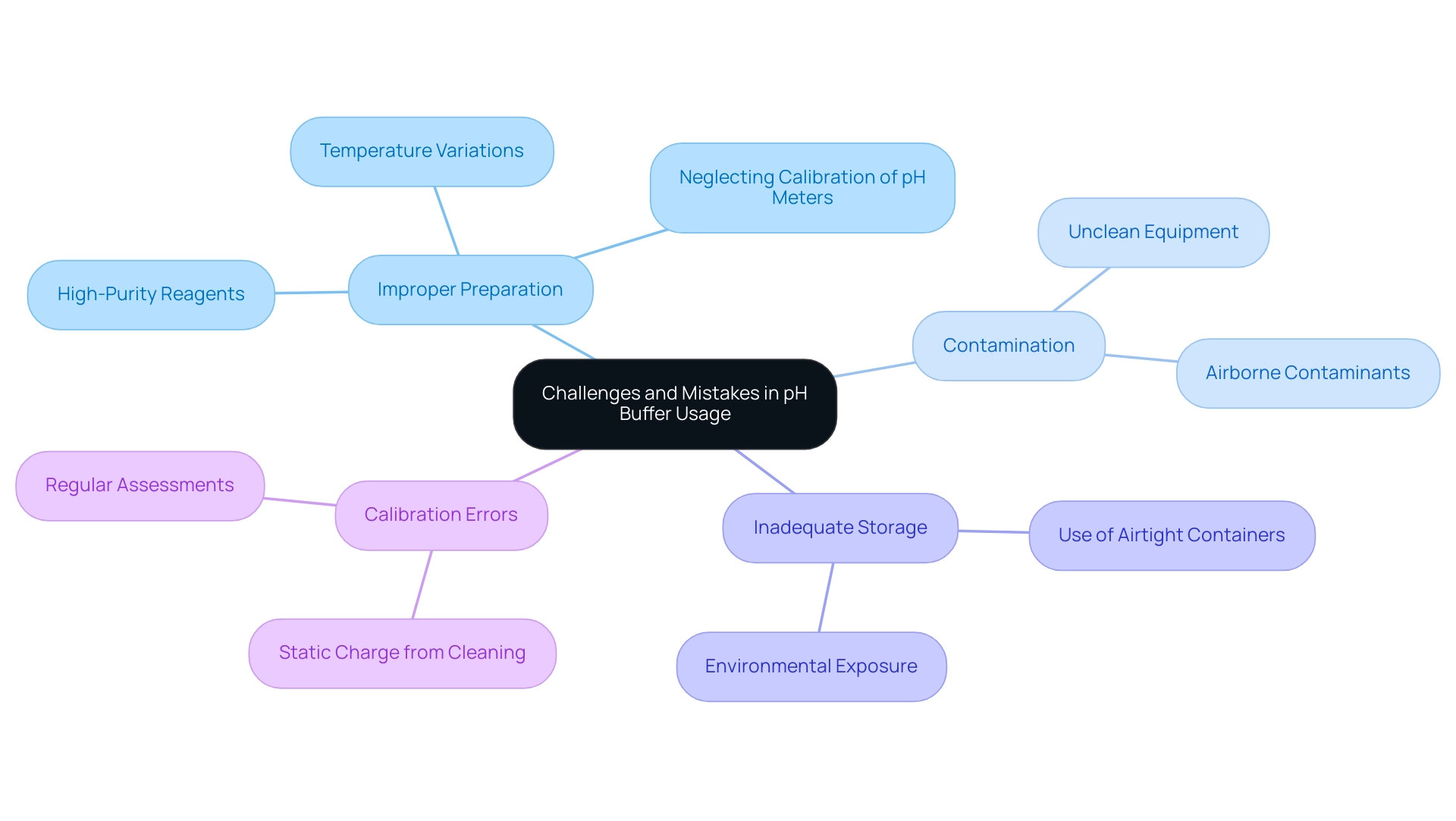
Common Challenges and Mistakes in pH Buffer Usage
Maintaining the integrity of pH buffer solutions presents several challenges, primarily stemming from improper preparation, contamination, and inadequate storage conditions. Using high-purity reagents is crucial when preparing solutions, as impurities can significantly compromise the stability of the pH buffer. Contamination can arise from various sources, including unclean equipment or exposure to the environment, underscoring the necessity of using clean, airtight containers for storage.
Moreover, facility staff must be well-trained to perform regular assessments on solution mixtures, ensuring they stay within their effective pH buffer range. Common mistakes in solution preparation often include neglecting to calibrate pH meters before use or failing to account for temperature variations, which can compromise the accuracy of the pH buffer and lead to inaccurate readings. Statistics indicate that a significant percentage of errors in the lab stem from these oversights, highlighting the need for stringent protocols. A study disclosed that up to 30% of laboratory mistakes are associated with improper preparation of pH buffer solutions, emphasizing the critical importance of adhering to established protocols.
Real-world examples demonstrate the effect of contamination on the effectiveness of the system. For instance, a study revealed that substances exposed to airborne contaminants exhibited shifts in pH, leading to unreliable experimental outcomes. Additionally, as noted by Hanna Instruments, "An electrode sends a voltage to your meter that is based on the pH buffer of the sample it is submerged in." Cleaning the pH glass can generate a static charge that disrupts the voltage reading of the electrode, emphasizing a common error that can impact solution stability.
By proactively tackling these challenges and applying best practices in solution preparation and storage, including the use of a pH buffer, facilities can improve the reliability of their results and uphold the integrity of their analytical processes. Furthermore, leveraging high-quality instruments, such as those offered by JM Science, including precision balances with a capacity of up to 64 kg and readability from 1 mg, can significantly enhance the accuracy of solution preparation. JM Science also offers advanced HPLC systems and titrators that are crucial for accurate analytical tasks, ensuring that facilities can maintain optimal levels of pH buffer in their mixtures.
JM Science's with prominent brands such as Agilent Technologies and Thermo Fisher Scientific guarantee that laboratories have access to high-quality resources that improve the reliability of their instrumentation, further aiding effective management. For more information on our products, visit JM Science's website.
The Role of Calibration and Maintenance in pH Buffer Effectiveness
Regular calibration of pH meters is essential for ensuring accurate measurements with a pH buffer, and it should be conducted frequently using standard solution alternatives. A two-point calibration is generally recommended, employing solutions that serve as a pH buffer to encompass the anticipated range of the samples being analyzed. This approach not only enhances measurement precision but also aligns with best practices in testing environments.
The Nernst equation provides a theoretical slope of 59 mV/pH for pH measurements, underscoring the scientific foundation for these calibration practices, which involve the use of a pH buffer. Furthermore, pH buffers necessitate periodic replacement to maintain their effectiveness, as their pH can drift over time due to factors such as evaporation, contamination, or degradation. Research indicates that neglecting the replacement of the intermediate solution can lead to significant inaccuracies in pH buffer readings, highlighting the importance of routine maintenance.
To illustrate, a case study on reference electrode stability underscores the critical role of stable reference potentials in pH measurement. It emphasizes the challenges posed by environmental factors and suggests improvements in electrode design to enhance stability and accuracy. Such insights reinforce the necessity of maintaining and a pH buffer to achieve dependable and consistent results in analysis.
Moreover, specialists recommend calibrating pH meters with a focus on the pH buffer range relevant to the samples being measured. K. L. Cheng notes that the potential measurement can be influenced by stirring or unstirring and the effects of double and triple layers, adding complexity to pH measurement. This targeted calibration strategy ensures that the measurements accurately reflect the true pH of the samples, thereby improving the reliability of experimental outcomes.
Additionally, rejuvenation methods for pH probes, such as soaking in manufacturer-recommended cleaning solutions followed by calibration, are crucial for maintaining the accuracy of pH meters. By adhering to these practices, institutions can significantly enhance the accuracy of their pH measurements, ultimately contributing to more robust scientific research.
Future Trends in pH Buffer Technology: Innovations and Developments
The future of pH stabilizing technology, particularly innovations in pH buffers, is on the cusp of transformative advancements. Formulations designed to enhance stability and minimize interference during experimental procedures are at the forefront of this evolution. Researchers are increasingly prioritizing the development of smart buffers—formulations that can adapt to changing conditions in real-time. This adaptability not only amplifies their effectiveness in dynamic settings but also ensures more accurate results across a wide range of applications.
Moreover, the integration of digital technologies, particularly IoT-enabled systems for pH buffer monitoring, is set to revolutionize the management of solutions within research facilities. These systems enable continuous monitoring and adjustment of pH levels, streamlining workflows and bolstering the reliability of experimental outcomes. Consequently, research facilities can anticipate heightened efficiency and precision in their analyses.
Current trends reveal a pronounced emphasis on innovative formulations tailored to specific experimental needs, reflecting a broader shift towards customization in scientific practices. The market for pH buffer stabilizing solutions is projected for substantial growth, with leading manufacturers investing heavily in research and development to produce next-generation pH buffer products that align with the evolving demands of scientific inquiry. A study examining major global producers in the pH buffer market from 2020 to 2025 underscores the commitment to innovation, highlighting the pivotal role of pH buffers in chemistry and establishing them as indispensable tools for enhancing research capabilities.
In this landscape, distinguishes itself through its unwavering commitment to quality and customer support, as detailed in the case study titled "Customer Support and Quality Commitment." This dedication significantly enhances the company's value proposition, particularly in the realm of pH buffer stabilization technology. Furthermore, the anticipated expansion of the Health Economics and Outcome Research (HEOR) Services Market, projected to reach $3.79 billion by 2031, underscores the critical importance of pH buffer solutions within the broader market context, linking them to advancements in laboratory capabilities.
Conclusion
In the intricate landscape of scientific research, the significance of pH buffers is paramount. These specialized solutions are essential for maintaining stable pH levels across various laboratory applications, from drug formulation in pharmaceutical settings to ensuring optimal conditions for enzyme activity in biochemical assays. By effectively neutralizing small amounts of acids or bases, pH buffers not only facilitate accurate measurements but also enhance the reliability of experimental outcomes.
The mechanisms behind pH stabilization reveal a dynamic equilibrium of weak acids and their conjugate bases. This equilibrium is crucial for mitigating pH fluctuations that can compromise both experimental integrity and the stability of pharmaceutical compounds. Furthermore, exploring different types of buffers—acidic, basic, and neutral—underscores the tailored approaches necessary for diverse laboratory requirements.
As the demand for precision in scientific endeavors continues to rise, the role of pH buffers evolves alongside advancements in technology. Innovations such as smart buffers and IoT-enabled monitoring systems pave the way for enhanced efficiency and adaptability in laboratory environments. However, challenges associated with buffer preparation, contamination, and maintenance remain pertinent, necessitating rigorous protocols to ensure the integrity and effectiveness of these solutions.
Ultimately, a comprehensive understanding of pH buffers is imperative for professionals in the scientific community. Recognizing their critical role in laboratory practices and staying abreast of technological advancements enables researchers to navigate the complexities of modern scientific exploration. As the field progresses, the continued emphasis on high-quality pH buffers will undoubtedly contribute to improved accuracy, reliability, and sustainability in laboratory results, shaping the future of scientific inquiry.




