Overview
Ensuring measurement accuracy and compliance with industry standards hinges on best practices for the calibration of pH sensors. Regular calibration is not merely a recommendation; it is a necessity. The selection of appropriate buffer solutions and strict adherence to manufacturer guidelines are critical steps that uphold sensor performance and prevent errors that could jeopardize experimental integrity. By implementing these practices, laboratories can significantly enhance the reliability of their measurements, ultimately fostering trust in their results.
Introduction
In the realm of scientific research and industrial applications, the significance of pH sensors is paramount. These advanced electrochemical devices play a crucial role in measuring the acidity or alkalinity of various solutions, which directly influences the accuracy of experimental results and product quality.
As technology continues to evolve, enhancing the capabilities of these instruments, it becomes essential for researchers and lab managers to understand the fundamentals of pH sensors, the importance of calibration, and best practices for maintenance.
This article explores the intricacies of pH sensors, offering insights into their operation, the necessity of regular calibration, and practical guidance for ensuring reliable measurements across diverse applications.
Understanding pH Sensors: Basics and Importance
pH instruments represent sophisticated electrochemical devices meticulously engineered to measure the acidity or alkalinity of a solution by detecting the concentration of hydrogen ions (H+). Typically, these devices comprise a glass electrode sensitive to H+ ions and a stable reference electrode. The output voltage generated by the device correlates directly with the pH level of the solution, emphasizing the necessity of understanding their operation for precise measurements.
Accurate pH measurements are paramount across various scientific disciplines, including chemistry, biology, and environmental science. In laboratory settings, pH devices serve as essential instruments for ensuring the reliability of experimental data. In industrial processes, they play a critical role in maintaining product quality and adherence to regulatory standards. Furthermore, in environmental monitoring, pH devices are vital for assessing water quality, soil health, and the overall ecosystem, underscoring their significance in sustainability efforts.
Recent advancements in pH measurement technology have markedly enhanced their performance and reliability. For instance, the sensitivity of TA-RGO pH devices is reported at −52.4 ± 0.7 mV/pH, demonstrating their precision in detecting minute changes in pH levels. Additionally, innovative advancements such as the integrated wearable chip reported by Niu's group in 2019 illustrate the potential for pH devices to monitor human sweat pH accurately, maintaining stability even under bending conditions. This aligns closely with results obtained from commercial pH meters, showcasing the increasing versatility of pH devices in both laboratory and real-world applications.
Real-world applications of pH measurement in environmental monitoring include their deployment in evaluating the health of aquatic ecosystems, where maintaining optimal pH levels is essential for the survival of aquatic life. Case studies indicate that accurate pH measurements can lead to improved management practices in water treatment facilities and agricultural settings, ultimately contributing to environmental sustainability.
Experts emphasize the importance of dependable pH measuring devices in laboratory environments, asserting that the calibration of pH sensors and a suitable standard measurement protocol, alongside advancements in stable solid-state reference components, are crucial for acquiring valid data. As Y.T. stated, "Additionally, a reasonable standard measurement protocol and the development of a more stable solid–state reference electrode should be introduced as soon as possible in the future—this is key to obtaining valid data."
This underscores the ongoing need for innovation and adherence to best practices in the calibration of pH sensors, ensuring that laboratories can meet the evolving demands of scientific research and environmental stewardship.
Moreover, understanding market dynamics and growth opportunities for pH devices is essential for pharmaceutical lab managers evaluating these tools. The addition of post-sales analyst support can further enhance the value proposition of pH devices, ensuring that laboratories receive the necessary guidance and assistance after their purchase.
The Importance of Calibrating pH Sensors for Accurate Measurements
The calibration of pH sensor devices is a fundamental practice that ensures their output aligns accurately with established pH standards. Routine adjustment is essential; the calibration of pH sensors is necessary to maintain the precision of pH measurements, which can decline over time due to factors such as electrode aging, contamination, and fluctuating environmental conditions. Research indicates that the lifespan of pH devices can vary from weeks to years, significantly influenced by usage conditions.
Without regular calibration of pH sensors, laboratories risk significant errors in their experimental results, which can adversely affect critical processes, including chemical reactions and biological assays. The consequences of neglecting pH sensor adjustment are profound. Inaccurate pH readings can lead to flawed experimental outcomes, making the calibration of pH sensors essential for maintaining research integrity and avoiding costly rework. For instance, a case study on adjustment modeling for proximal soil sensing in precision agriculture demonstrated that traditional adjustment methods, which often necessitate extensive field sampling, can be both time-consuming and costly.
By employing neighborhood or farm models, researchers were able to reduce the number of reference samples required for adjustment by approximately 74% and 87%, respectively, while preserving model performance. This underscores the efficiency gains achievable through effective calibration of pH sensor strategies. Furthermore, the impact of sensor aging on pH measurements cannot be overstated. As electrodes age, their response to pH changes can become sluggish, leading to inaccurate readings that can distort experimental data. Routine calibration of pH sensors not only enhances measurement precision but also extends the longevity of pH devices.
Implementing intelligent device management (ISM) technology can further enhance longevity by providing predictive diagnostics that monitor health and maintenance requirements. As noted, "Our ISM™ technology features predictive diagnostic tools including an Adaptive Calibration Timer," which emphasizes the importance of proactive maintenance. Statistics reveal that numerous laboratories do not adhere to suggested adjustment frequencies, jeopardizing the reliability of their results. JM Science Inc. boasts over 780 accreditations in 28 metrological and test domains, reinforcing its credibility and expertise in measurement and sensor technology. Establishing a routine schedule for the calibration of pH sensors is essential for maintaining the integrity of scientific data and ensuring compliance with industry standards.
By prioritizing the calibration of pH sensors, laboratories can safeguard their research outcomes and uphold the highest standards of accuracy in their measurements.
Step-by-Step Guide to Calibrating pH Sensors
- Prepare Calibration Solutions: Select at least two pH solutions that encompass the expected pH range of your samples, such as pH 4 and pH 7. Utilizing fresh materials that have been stored appropriately is essential for ensuring accuracy.
- Rinse the pH sensor: Thoroughly rinse the pH sensor with deionized water to eliminate any contaminants from prior measurements, which could affect the calibration results.
- Immerse in Solution: Submerge the probe in the first solution (e.g., pH 7) and allow it to stabilize. Monitor the reading until it becomes consistent, which typically takes a few moments.
- Adjust the calibration of the pH sensor by following the manufacturer's instructions to calibrate the meter to the known pH value of the solution. This step is crucial for ensuring that the calibration of the pH sensor provides accurate measurements.
- Repeat with Second Buffer: After rinsing the electrode again, immerse it in the second buffer (e.g., pH 4) and repeat the stabilization and adjustment process. This two-point adjustment is vital for the calibration of the pH sensor to enhance accuracy across the pH scale. Notably, Atlas Scientific recommends a 3-point adjustment technique for the calibration of pH sensors to achieve the most precise results.
- Verify Adjustment: Once the adjustment with both buffers is complete, validate the accuracy by measuring a third buffer solution. This step ensures that the calibration of the pH sensor yields readings that are consistent and reliable.
- Document Adjustment: Record all adjustment results and any modifications made during the process. This documentation is important for compliance and future reference, helping to maintain the integrity of laboratory measurements, particularly concerning the calibration of the pH sensor, which is necessary before first use, after long storage, after cleaning or electrolyte replacement, after measuring in strong solutions, and when very accurate measurements are needed. Recognizing that shifting one pH unit on the scale relates to a variation in voltage of 59 mV emphasizes the accuracy needed in adjustment. Furthermore, fluctuations in the output of pH sensors may occur due to elements like offset, slope, and span, which underscores the importance of the calibration of pH sensors to ensure precise measurements throughout the pH spectrum.
Choosing the Right Buffer Solutions for Calibration
Selecting the appropriate solution is essential for the calibration of pH sensors and achieving effective pH adjustment. The choice of buffers should be determined by the expected pH range of the samples being measured for sensor calibration. For general laboratory applications, pH 4 and pH 7 solutions are commonly used for two-point adjustments.
When dealing with samples that may be more acidic or alkaline, it is advisable to incorporate additional solutions, such as pH 10, to aid in the calibration of the pH sensor and ensure accuracy across a broader range. The calibration of the pH sensor is critical, as the freshness of solution preparations significantly impacts measurement precision when outdated mixtures are employed. As noted by the Technical Department, this indicates a 100 to 1,000 times increase in H+ ion concentration, highlighting the vital importance of precise adjustment.
Scientific experts indicate that the variability from adjustment solutions typically falls within ± 0.02 pH units, underscoring the necessity of calibrating pH sensors with reliable and fresh mixtures. Consistent monitoring of expiration dates and timely replacement of solutions is a best practice that laboratories should adopt to maintain accuracy during pH sensor calibration. When selecting solution options, it is imperative to consult the manufacturer's guidelines for specific recommendations tailored to your equipment and applications.
This approach ensures that the calibration process for pH sensors adheres to the highest standards of accuracy and compliance. Case studies reveal that laboratories effectively utilizing pH 4 and pH 7 solutions for standardization, in conjunction with the calibration of pH sensors, achieve consistent and reliable results, emphasizing the importance of appropriate solution selection in attaining accurate measurements. Furthermore, JM Science Inc. offers NIST-certified pH standard solutions in 500ml and 20ml sizes, available individually and in sets, with prices ranging from $27.00 to $89.00.
The case study titled 'Manage Your Sachet Stock' illustrates how dispenser boxes can assist laboratories in efficiently tracking their pH buffer inventory, ensuring a steady supply of solutions for adjustments, which is crucial for the calibration of pH sensors to maintain precise measurements.
Troubleshooting Common pH Calibration Issues
Common issues encountered during the calibration of pH sensors can significantly impact measurement accuracy and laboratory efficiency. Addressing these challenges is crucial for maintaining compliance and ensuring reliable results.
- Drifting Readings: Fluctuating pH measurements frequently suggest contamination or impairment to the sensor. Routine cleaning of the component and recalibration can alleviate this issue. It is essential to monitor the condition of the device; neglecting maintenance can lead to increased costs from additional testing required due to uncalibrated equipment. Each additional test due to uncalibrated equipment represents lost resources, emphasizing the importance of diligent maintenance.
- Inconsistent Readings: Fluctuating measurements may arise from using expired or improperly stored solution mixtures. Ensuring that the solutions are fresh and that the conductor is completely submerged in the liquid is essential. Allowing adequate time for the readings to stabilize can also help achieve consistent results. It is advised to adjust all pH meters utilizing the same certified reference solution and to adhere to the same adjustment methods to ensure precision.
- Calibration Failure: A failure to calibrate can arise from using incorrect buffer solutions or a faulty sensor. Routine inspections of functionality are advised, and substituting worn or damaged components is essential to prevent calibration problems. Utilizing diagnostic tools, such as Hanna Instruments' CAL Check™, can assist in verifying sensor performance. It is especially crucial to avoid utilizing old or expired devices, which can become less responsive over time.
- Temperature Effects: Temperature variations can significantly influence pH readings. Employing temperature-compensated buffers or adjusting the calibration process according to the solution's temperature is essential for accurate measurements. This practice is particularly important in specialized applications, such as those involving high temperatures or fermentation broths, where separating the reference sensor from the indicator sensor is advised to maintain accuracy, as noted by K. L. Cheng.
- Specialized Probes for Challenging Samples: In situations involving viscous or solid materials, utilizing specialized probes designed to prevent clogging is essential. Adhering to manufacturer guidelines for these components improves measurement precision, guaranteeing dependable outcomes even in difficult circumstances. A case study titled "Handling pH Measurements for Viscous or Solid Samples" illustrates the importance of using specialized electrodes and adhering to manufacturer guidelines for accurate measurements.
By understanding and addressing these common issues related to the calibration of pH sensors, laboratories can improve their measurement accuracy and operational efficiency, ultimately leading to better compliance and reduced costs.
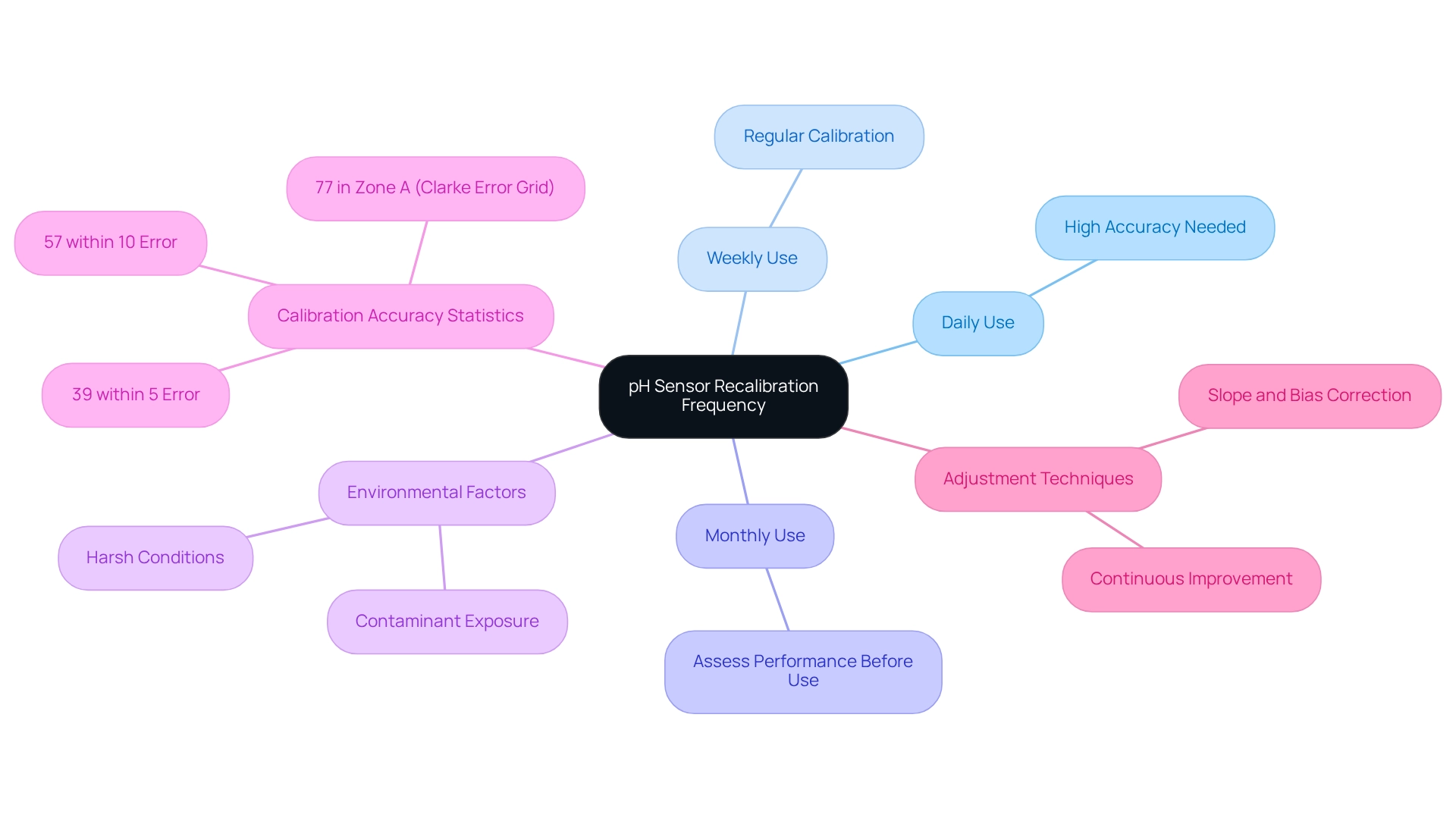
How Often Should You Recalibrate Your pH Sensor?
The frequency of calibration for pH sensors is influenced by multiple factors, including the nature of the samples being analyzed, the frequency of device usage, and the precision required for specific applications. To ensure optimal performance, consider the following general guidelines for recalibration:
- Daily Use: For applications that demand high accuracy, recalibrating the device before each use is advisable.
- For equipment that is utilized regularly but not daily, a weekly calibration of the pH sensor is recommended to maintain accuracy.
- For instruments that are infrequently used, a monthly calibration may suffice; however, assessing the device's performance prior to use is crucial.
In laboratory environments characterized by harsh conditions or exposure to contaminants, more frequent calibration of pH sensors may be necessary. Recent studies indicate that 39% of measurement values were within a 5% range of relative error, while 57% fell within a 10% range. This underscores the importance of pH sensor calibration in maintaining measurement accuracy. Notably, employing the Clarke error grid approach revealed that 77% of the evaluated data pairs were located in zone A, suggesting clinically acceptable outcomes. This further emphasizes the essential role of calibration in achieving dependable measurements.
Moreover, case studies on the frequency of pH instrument readjustment highlight that modifications in adjustment protocols can significantly influence the reliability of outcomes, particularly in pharmaceutical environments where accuracy is vital. For instance, slope and bias adjustment methods used with potentiometric array devices illustrate the challenges and solutions pertinent to pH measurement, showcasing the necessity for continuous improvement in adjustment techniques.
Experts recommend that laboratories establish a recalibration timetable for pH sensors based on usage frequency and environmental factors. This approach ensures adherence to industry standards and enhances the overall quality of analytical outcomes. As Alisa Rudnitskaya from the Center for Environmental and Marine Studies notes, signal standardization involves utilizing a relationship between measurement responses at the time of calibration and new conditions, which is essential for correcting data obtained from unknown samples. This highlights the critical importance of recalibration techniques in maintaining measurement accuracy.
Best Practices for Maintaining pH Sensors and Probes
To ensure the effective upkeep of pH instruments and probes, it is crucial to implement the following best practices:
- Regular Cleaning: Frequent cleaning of the device is essential to eliminate deposits or contaminants that may interfere with accurate readings. Utilize cleaning solutions that are suitable for the specific type of contamination encountered, as this will help maintain sensor performance.
- Proper Storage: Store the device in an appropriate solution designed to maintain its hydration. Avoid using distilled water for storage, as this can lead to harm and diminished performance of the component over time.
- Check for Damage: Conduct regular inspections of the component to identify any signs of wear or damage. If the electrode shows significant aging or a decline in performance, it should be replaced promptly to ensure continued accuracy in measurements.
- Follow Manufacturer Guidelines: Adhering to the manufacturer's recommendations for maintenance and care is vital for achieving optimal performance and extending the lifespan of the device. This includes understanding the average lifespan of pH devices, which typically ranges from 6 months to 2 years, depending on usage and maintenance.
- Utilize Diagnostic Tools: Implement predictive diagnostic instruments that can signal when a pH device requires adjustment or replacement. According to ISM's predictive diagnostic tools, these can assess factors such as process temperature, pH value, and slope of the measurement device, allowing for proactive maintenance and minimizing downtime.
- Stay Informed on Technology Advancements: Keep abreast of developments in digital measurement technology, which offer enhanced functionalities for offline adjustments and provide diagnostic data. The transition from analog to digital sensors can lead to improved precision and operational efficiency, as evidenced by case studies showcasing the benefits of digital technology in laboratory environments.
By adhering to these best practices, laboratory managers can ensure the reliability and accuracy of pH measurements through the calibration of pH sensors, ultimately supporting compliance and quality within their analytical procedures.
Ensuring Compliance: Regulatory Standards for pH Calibration
Compliance with regulatory standards is essential for laboratories conducting pH measurements, especially in the pharmaceutical and environmental sectors, where the calibration of pH sensors is critical. Organizations such as the United States Pharmacopeia (USP) and the Environmental Protection Agency (EPA) set forth guidelines that govern pH adjustment procedures. Adhering to these standards not only guarantees the accuracy of measurements but also preserves the integrity of laboratory data.
Key compliance aspects include:
- Adjustment Frequency: Regulatory authorities recommend that internal adjustments be performed at least once daily using certified reference buffer standards. External adjustments should typically occur annually. Each quality control laboratory must establish its adjustment intervals based on its quality management system to ensure consistent accuracy. A recent case study underscores the importance of defining these intervals for maintaining compliance and reliability in testing outcomes.
- Use of Certified Standards: Employing certified reference solutions is vital for achieving high accuracy and traceability in pH measurements. These buffers can be traced to certified reference materials, enhancing the reliability of measurement results. Documentation: Maintaining comprehensive records of measurement procedures, results, and any modifications is crucial for adhering to quality assurance protocols. This documentation serves as a reference for audits and guarantees transparency in laboratory operations.
- Training: It is imperative that staff responsible for adjustments receive adequate training and possess a thorough understanding of regulatory requirements. This knowledge is essential for ensuring compliance and that all measurement processes align with established standards. Recent statistics reveal that compliance requirements for pH measurement in laboratories are becoming increasingly stringent, with some proficiency testing goals tightening to 40% in 2024. As Sharon S. Ehrmeyer, Ph.D., articulates, 'When results of controls and calibration materials fail to meet the laboratory's established criteria for acceptability, all patient test results obtained in the unacceptable test run or since the last acceptable test run must be evaluated to determine if patient test results have been adversely affected, and the laboratory must take remedial action necessary to ensure the reporting of accurate and reliable patient test results.' Laboratories that comply with USP and EPA guidelines not only bolster their operational credibility but also enhance the overall reliability of their testing outcomes.
By implementing these best practices, laboratories can ensure that their pH measurement processes, particularly the calibration of pH sensors, are both accurate and compliant with regulatory standards.
Conclusion
The exploration of pH sensors underscores their critical role in scientific research and industrial applications, serving as essential tools for measuring acidity and alkalinity. A comprehensive understanding of how these sensors operate—including the necessity of regular calibration and maintenance—is vital for ensuring reliable measurements. Recent advancements in pH sensor technology significantly enhance their performance, rendering them indispensable across various fields, from environmental monitoring to pharmaceuticals.
Regular calibration transcends mere procedural necessity; it is crucial for preserving the accuracy of pH measurements. The repercussions of neglecting this fundamental practice can result in substantial errors, jeopardizing research integrity and product quality. By adhering to a structured calibration schedule and implementing effective strategies, laboratories can mitigate risks and uphold the reliability of their results.
Best practices for maintaining pH sensors—such as proper storage, routine cleaning, and the use of diagnostic tools—further enhance the longevity and accuracy of these instruments. Compliance with regulatory standards is equally imperative, ensuring that laboratories meet established guidelines and maintain the integrity of their data.
In conclusion, the effective use and maintenance of pH sensors are essential for achieving accurate measurements and advancing scientific inquiry. By prioritizing calibration, adhering to best practices, and comprehending the regulatory landscape, laboratories can confidently equip themselves to meet the challenges posed by modern research and industry demands.




