Overview
This article serves as a comprehensive step-by-step guide on calibrating a pH meter, highlighting the essential nature of this process for achieving accurate and reliable measurements in laboratory environments. It details the calibration procedure utilizing standard buffer solutions, outlines best practices for maintenance, and discusses the serious implications of neglecting calibration. Such insights underscore the critical role calibration plays in scientific research and quality control, prompting readers to recognize the necessity of this fundamental practice.
Introduction
In the realm of scientific research and quality control, the significance of pH meters is paramount. These sophisticated instruments, engineered to measure the acidity or alkalinity of solutions, play a crucial role across various applications, from pharmaceuticals to environmental monitoring. With a pH scale that spans from 0 to 14, mastering the accurate utilization and maintenance of these devices is essential for achieving reliable results. As technology progresses, the functionality of pH meters evolves, enhancing their user-friendliness and efficiency. Nevertheless, the accuracy of these measurements is contingent upon proper calibration and maintenance practices.
This article explores the intricacies of pH meters, providing insights into their operation, the critical importance of calibration, and best practices for ensuring their longevity and precision. Whether in a bustling laboratory or a field study, mastering pH measurement techniques is vital for safeguarding research integrity and public health.
Understanding pH Meters: Basics and Functionality
is a sophisticated electronic device specifically engineered to measure the acidity or alkalinity of a liquid, quantified as pH. This device consists of two essential components: a pH-sensitive electrode and a reference electrode. Together, these electrodes assess the hydrogen ion concentration in the liquid, facilitating precise pH readings.
The pH scale spans from 0, indicating a highly acidic solution, to 14, representing a highly alkaline one, with 7 denoting neutrality. Understanding the interaction between these components is crucial for achieving accurate measurements. Any malfunction or miscalibration can lead to significant errors, potentially impacting experimental outcomes. In controlled environments, where accuracy is paramount, even slight variations in pH measurements can result in erroneous data and conclusions.
Recent advancements in pH measuring technology have enhanced their functionality and reliability. frequently feature digital displays, automatic temperature compensation, and advanced calibration options, rendering them more user-friendly and efficient. Statistics reveal that a substantial number of laboratories rely on pH devices for various applications, underscoring their significance in scientific research and quality assurance processes.
Notably, 37.7 million Indians are affected by waterborne illnesses each year, highlighting the essential role of pH instruments in environmental monitoring and public health.
The practical applications of pH instruments are diverse, encompassing fields such as pharmaceuticals, environmental oversight, and food safety. In pharmaceutical laboratories, maintaining the correct pH is vital for drug formulation and stability, while in environmental studies, pH measurements can indicate water quality and contamination levels. 'JM Science Inc.' emphasizes high-performance HPLC components, demonstrating its commitment to providing precision instruments that support these critical scientific practices.
Expert opinions underline the importance of understanding pH device functionality. Dr. Surat P., a Ph.D. in Cell Biology and Mechanobiology, asserts that familiarity with the device's components and operational principles not only aids in troubleshooting but also enhances overall efficiency in the workspace. By ensuring that lab managers are well-versed in the calibration of pH meters, they can better maintain these devices, ultimately leading to more reliable results in their scientific endeavors.
'JM Science Inc.'s dedication to quality and customer support, coupled with its strategic focus on innovation, positions it as a valuable partner for research facilities seeking to enhance their analytical capabilities.
The Importance of Calibrating Your pH Meter
Calibration is a fundamental practice essential for understanding how a pH meter is calibrated, ensuring the accuracy and reliability of pH devices in pharmaceutical settings. Over time, electrodes can experience drift, leading to significant inaccuracies in readings. Frequent adjustments are crucial to comprehend how a pH meter is calibrated, synchronizing the device's output with recognized standards, typically employing buffer solutions at specific pH levels, such as pH 4, 7, and 10.
This meticulous process not only enhances measurement accuracy but also safeguards the integrity of laboratory results. Neglecting proper adjustment can have dire consequences, potentially compromising research outcomes and violating industry regulations. For instance, a study on standardized buffers for consistent results underscores the importance of calibrating pH meters with at least three standard buffers, which is vital for accounting for variations in sample characteristics. Such practices yield more dependable pH measurements, enabling users to trust the precision of their outcomes across various samples and conditions.
Expert insights further highlight the significance of adjustment. K L Cheng from the University of Missouri-Kansas City emphasizes the necessity of addressing interference from phosphate buffers, illustrating how incorrect adjustments can distort results. Statistics indicate that while a sensitivity of pH 0.01 is reasonable for adjustment, a sensitivity of pH 0.001 is seldom pursued, signifying that even slight inaccuracies can have substantial effects on research results.
Moreover, the development of new technologies, including advanced reference electrodes and conducting wires to replace conventional salt bridges, presents opportunities for more precise and stable pH measurements. These innovations exemplify the continuous evolution in , emphasizing the need for facilities to stay informed about the latest adjustment protocols. JM Science Inc. is committed to providing these advanced measurement technologies and fostering strong partnerships with leading manufacturers, ensuring that laboratories have access to the best tools for precise measurements.
Integrating robust measurement protocols into routine maintenance is not merely a best practice; it is an essential aspect of laboratory operations. By prioritizing regular calibration, lab managers can ensure the accuracy of their pH measurements and understand how a pH meter is calibrated, ultimately enhancing the reliability of their research and compliance with regulatory standards.
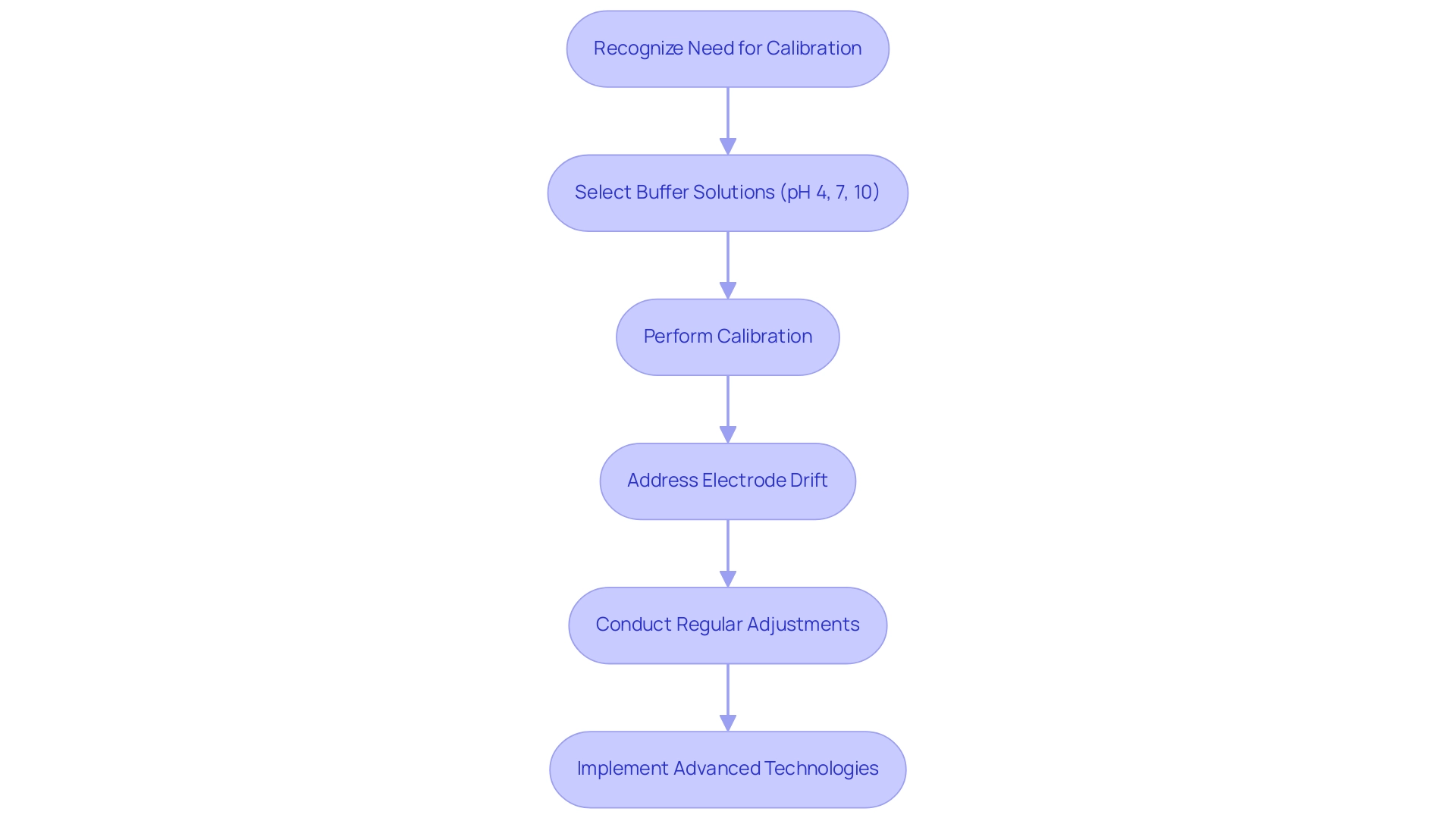
Step-by-Step Calibration Process for pH Meters
- Prepare Calibration Mixtures: Begin by gathering fresh buffer liquids at pH 4, 7, and 10. Ensure these solutions are uncontaminated and at room temperature to guarantee accurate readings.
- Turn on : Power up the pH meter and allow it to stabilize for a few minutes. This step is crucial for obtaining reliable measurements.
- Rinse the Electrode: Thoroughly rinse the pH electrode with distilled water to eliminate any residues from previous measurements, which could impact the accuracy of the settings.
Immerse in pH 7 Buffer: Submerge the electrode in the pH 7 buffer solution. Wait for the reading to stabilize, which typically takes a few moments, then press the 'Calibrate' button to initiate the calibration process. - Adjust Calibration: If the displayed reading does not match the buffer value (7.00), adjust it accordingly. This step is essential for ensuring the meter's accuracy at neutral pH.
- Rinse and Repeat: Rinse the electrode again with distilled water, then immerse it in the pH 4 buffer solution. Repeat the stabilization and adjustment process to ensure precision at this acidic level.
- Final Adjustment: For improved precision across a wider pH range, consider repeating the adjustment with the pH 10 buffer. This three-point adjustment method is recommended for optimal performance.
Store the Electrode: After completing the adjustment, store the electrode in a suitable storage solution to maintain its condition and prolong its lifespan.
In practice, the average time taken to adjust how a pH meter is calibrated is approximately 10 to 15 minutes, depending on the complexity of the adjustment process and the number of buffer solutions used. Effective calibration methods, including how a pH meter is calibrated, are essential, as emphasized by managers who stress the significance of upholding high-quality standards in their measurements. As noted by Westgard JO, the Medical Director is ultimately responsible for defining the quality requirement and the limit for acceptable performance.
By adhering to these best practices, laboratories can significantly reduce error propagation and enhance the reliability of their analytical results. Moreover, JM Science Inc.'s dedication to quality and customer assistance, as demonstrated in the case study named 'Customer Support and Quality Commitment,' emphasizes the significance of effective adjustment practices in attaining precise and dependable results.
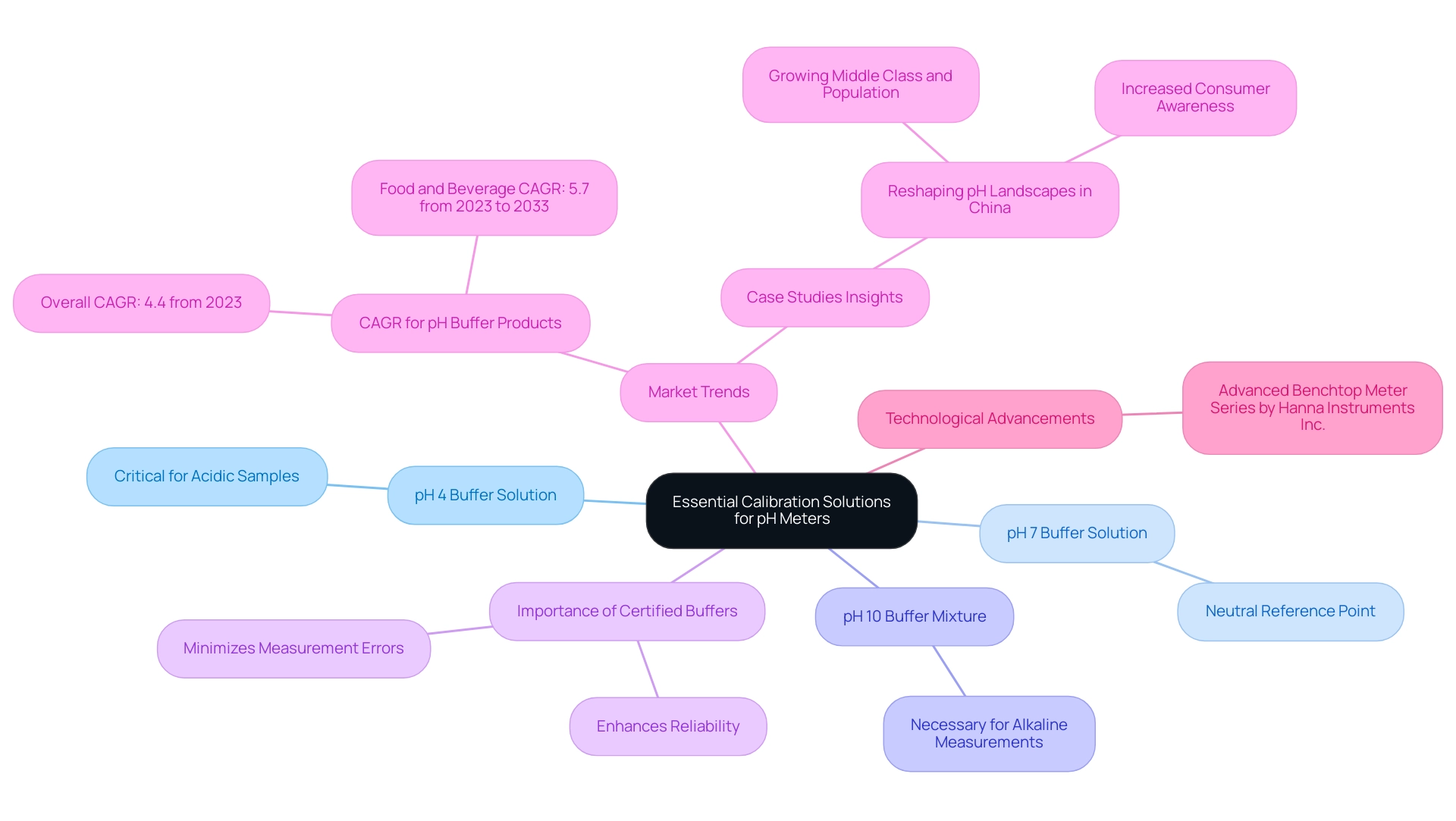
Essential Calibration Solutions: What You Need
To achieve , utilizing the following calibration solutions is essential:
- pH 4 Buffer Solution: This solution is critical for measuring acidic samples, providing a baseline for low pH readings.
- pH 7 Buffer Solution: Serving as the neutral reference point, this buffer is indispensable for most calibration processes, ensuring that the meter can accurately assess neutral pH levels.
- pH 10 Buffer Mixture: This mixture is necessary for calibrating alkaline measurements, allowing for precise readings in higher pH ranges.
Ensuring that these buffer mixtures are fresh and stored under appropriate conditions is crucial to preserve their chemical integrity. Utilizing certified buffer substances from reputable suppliers not only guarantees accuracy but also enhances the reliability of your measurements.
Recent studies emphasize the efficiency of certified buffer mixtures in adjustment processes. High-quality buffers can greatly minimize measurement errors. For instance, the market for pH buffer products is expected to expand, with a compound annual growth rate (CAGR) of 4.4% from 2023, indicating the rising need for dependable measuring instruments across various sectors, such as pharmaceuticals and food and beverage. Furthermore, the CAGR for food and beverage buffering agents is anticipated to be 5.7% from 2023 to 2033, highlighting broader market trends and the significance of certified options in these sectors.
Moreover, case studies, such as 'Reshaping pH Landscapes: Expanding Horizons in the China Buffering Agents Market,' demonstrate the increasing significance of quality in measurement tools. As consumer awareness about product quality grows, the demand for certified measurement services is expected to rise, propelled by technological progress and governmental initiatives.
In summary, the importance of understanding how pH meters are calibrated with certified adjustment methods cannot be overstated. These methods ensure accurate readings and elucidate the calibration process, contributing to the overall quality and reliability of laboratory analyses. Additionally, with advancements in pH measurement technology, such as the recent introduction of the Advanced Benchtop Meter Series by Hanna Instruments Inc., the significance of high-quality adjustment solutions continues to grow.
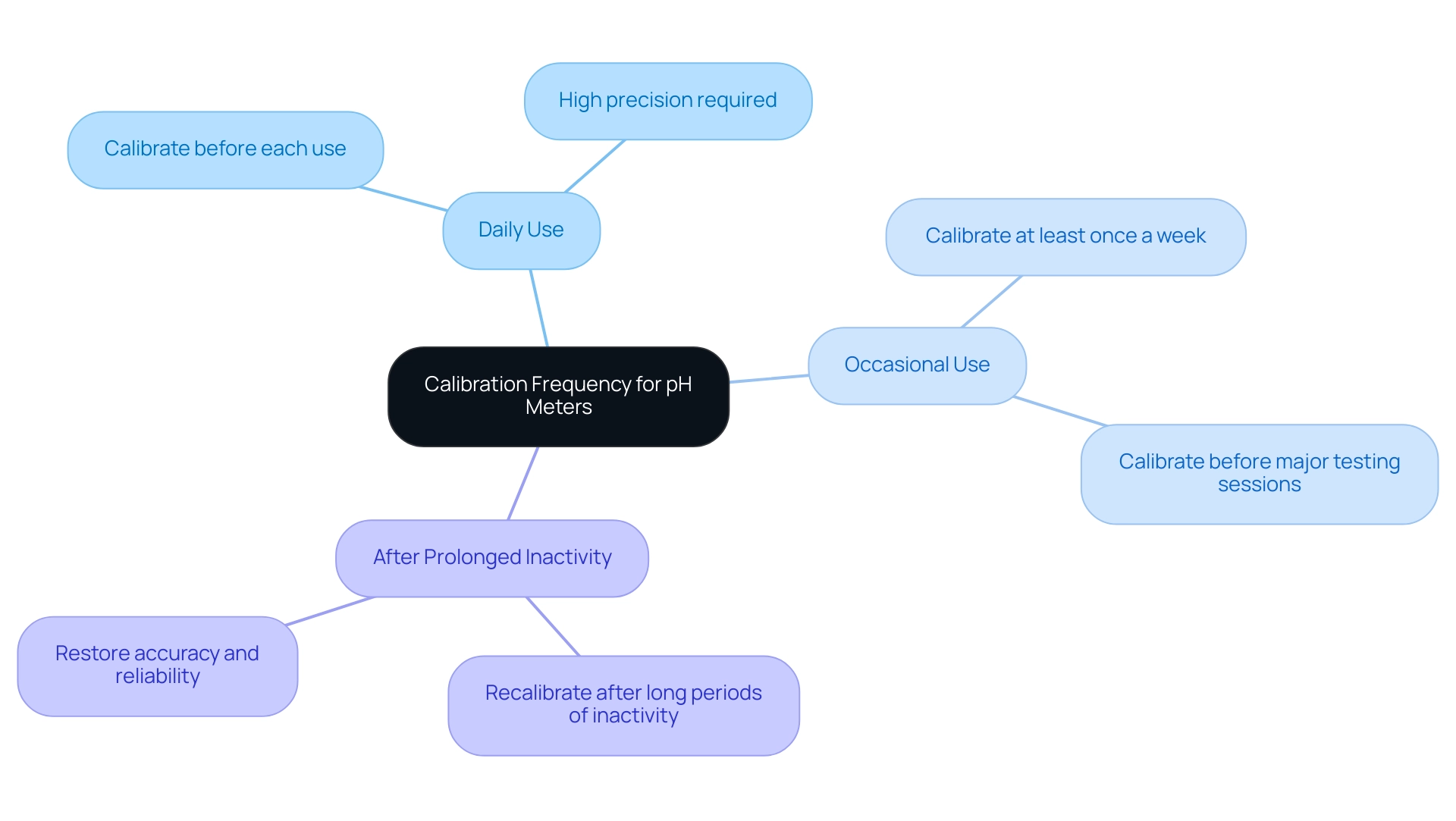
How Often Should You Calibrate Your pH Meter?
The frequency of calibration for [pH devices](https://jmscience.com) is significantly influenced by various factors, including the nature of the samples being analyzed and the frequency of device utilization. To ensure optimal accuracy, the following guidelines are recommended:
- Daily Use: For applications requiring high precision, it is imperative to calibrate the pH meter before each use. This practice is crucial in environments where measurement accuracy is paramount, such as pharmaceutical laboratories.
- Occasional Use: If the pH device is utilized less frequently, calibration should occur at least once a week or prior to any major testing sessions. This ensures that the instrument remains reliable and that measurements are consistent.
- After Prolonged Inactivity: Always recalibrate the meter after it has been idle for an extended period. This step is vital to restoring accuracy and reliability, particularly following long breaks between uses.
Regular adjustment is essential for understanding how pH meters are calibrated to maintain the integrity of laboratory results. A study on calibration practices highlights that employing a two-point or multi-point calibration method significantly enhances measurement accuracy. It is also advisable to utilize fresh buffer solutions, ideally from single-use packets, especially when calibrating less frequently.
Expert recommendations suggest that the ideal sensitivity of a pH device is approximately 0.0017 pH, based on a 60 mV/pH slope. This level of sensitivity underscores the necessity for careful adjustment practices. Moreover, technical experts stress that understanding how pH meters are calibrated is essential for precise adjustment, as highlighted in a case study examining the pH measurement adjustment process.
K. L. Cheng suggests that "a new type of reference electrode and the use of a conducting wire to replace the salt bridge are suggested for more accurate and stable pH measurements."
Furthermore, the assay and characteristics of tryptophan oxidative decarboxylase indicate optimal activity between pH 7.2 and 9.2, emphasizing the significance of in experimental applications.
In summary, adhering to these frequency guidelines not only enhances measurement precision but also reinforces the overall dependability of analyses, ultimately contributing to improved research results and better quality control in scientific endeavors.
Troubleshooting Calibration Issues: Common Problems and Solutions
[Calibration issues related to pH meters](https://pmc.ncbi.nlm.nih.gov/articles/PMC11206179) can significantly impact test results and, consequently, patient safety. Understanding these prevalent problems and their solutions is crucial for laboratory managers seeking to enhance reliability in their analytical processes.
Drifting Readings: Unstable readings often indicate a dirty or improperly hydrated electrode. To resolve this, rinse the electrode with distilled water and follow the calibration steps for the pH meter. Regular maintenance is essential, as drifting can lead to analytical biases that may cost research facilities between 60 million to 199 million USD each year due to inaccurate results.
Inconsistent Outcomes: Observing substantial differences between adjustments necessitates examining the buffer substances for contamination or expiration. Always utilize fresh buffers to ensure accuracy. A case study in medical settings highlights the importance of high-quality buffers, reinforcing that neglecting this aspect can lead to unreliable measurements.
Slow Response Time: A sluggish electrode response may indicate the need for cleaning or soaking in an appropriate storage solution. Ensure the electrode is not dried out, as this can severely affect its performance. Lab supervisors have noted that addressing this issue promptly can prevent measurement failures that disrupt lab operations.
Adjustment Failure: If the meter fails to adjust, first check the connections and ensure it is set to the correct mode for adjustment. Persistent issues may indicate that the electrode requires replacement. Expert insights reveal that a significant percentage of facilities encounter measurement errors, often due to overlooking these basic troubleshooting steps.
As Miller et al. noted, "An essential attribute for stable metrological traceability is consistency and stability for all elements in the measurement hierarchy..." This underscores the critical nature of maintaining . Furthermore, continuous quality control monitors the analytical process to ensure reliable analysis results, ultimately contributing to patient safety.
By proactively addressing these common measurement issues, laboratory managers can significantly enhance the reliability of their analytical processes, thereby improving patient care and safety.
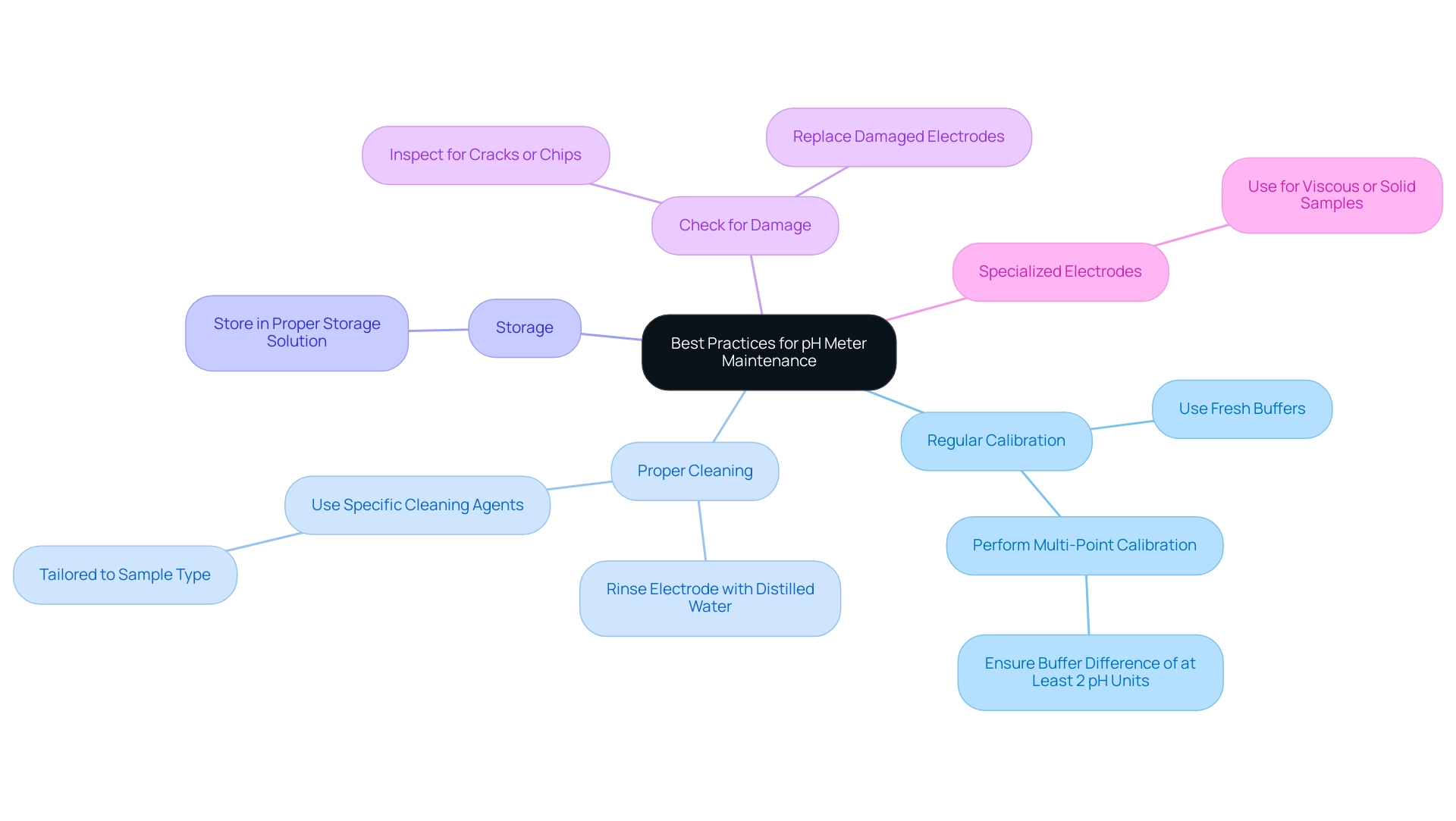
Best Practices for Maintaining Your pH Meter
To ensure the longevity and accuracy of your pH meter, it is essential to adhere to the following best practices:
- Regular Calibration: Calibration should be performed regularly, ideally before each use or at least once a week, depending on the frequency of use. Employing fresh, unexpired buffer preparations is crucial, as utilizing expired buffers can result in flawed adjustments. For multi-point calibration, ensure that the difference between buffer liquids is at least 2 pH units to enhance accuracy.
- Proper Cleaning: After each use, rinse the electrode with distilled water to remove any residual samples. Periodically, clean the electrode with a cleaning agent specifically designed for the type of sample being tested. This practice not only removes contamination but also enhances the electrode's performance and reliability, leading to more accurate pH readings. A case study on cleaning recommends utilizing specific cleaning agents tailored to the type of sample being tested to effectively remove contamination.
- Storage: When not in use, store the electrode in a proper storage solution to keep it hydrated. This is essential for maintaining the integrity of the glass membrane and ensuring optimal performance.
- Check for Damage: Regularly inspect the electrode for signs of wear or damage. Look for cracks, chips, or any other irregularities that could affect measurement accuracy. If any damage is detected, replace the electrode promptly to avoid compromising your data quality.
- Specialized Electrodes: For viscous or solid samples, utilize specialized electrodes designed for such applications to ensure accurate measurements.
Implementing these practices is vital for understanding how the pH meter is calibrated to ensure the accuracy and reliability of your pH measurements. Regular preventive maintenance, including routine checks and cleaning, significantly enhances the lifespan of pH instruments, ensuring compliance and efficiency in laboratory processes. A case study on preventive maintenance highlighted that users who engaged in regular maintenance saw improved accuracy in their pH measurements, ultimately leading to consistent product quality.
As Hanna Instruments states, "That's why we've dedicated our blog as a helpful resource for you to use!" By following these guidelines, lab managers can ensure their pH meters remain in optimal condition, supporting precise analytical results.
Conclusion
The intricate workings of pH meters underscore their vital role in scientific research and quality control. A thorough understanding of their basic functionality, the importance of calibration, and best maintenance practices is essential for achieving accurate and reliable measurements. These devices, equipped with advanced technology, are indispensable across various fields, from pharmaceuticals to environmental monitoring, where even minute discrepancies can lead to significant consequences.
Regular calibration is paramount to maintaining the integrity of pH measurements. Adhering to established protocols and utilizing fresh, certified buffer solutions ensures that laboratory results are both accurate and compliant with industry standards. Furthermore, troubleshooting common calibration issues can help mitigate potential errors, safeguarding research outcomes and enhancing patient safety.
Implementing best practices for the care and maintenance of pH meters not only prolongs their lifespan but also enhances the precision of analytical results. By prioritizing these practices, laboratories can significantly improve the quality and reliability of their data, ultimately contributing to more effective research and public health initiatives.
In conclusion, mastering the use of pH meters through proper understanding, calibration, and maintenance is essential for any laboratory professional. As technology continues to advance, staying informed and adhering to best practices will ensure that pH meters remain reliable tools in the pursuit of scientific accuracy and quality assurance.




