Overview
This article presents an in-depth overview of ten applications of Near Infrared (NIR) technology in pharmaceutical laboratories, underscoring its pivotal role in optimizing critical processes such as:
- Quality control
- Moisture analysis
- Drug formulation development
- Environmental monitoring
Each application illustrates how NIR methods significantly enhance efficiency, accuracy, and adherence to regulatory standards, thereby bolstering the safety and effectiveness of pharmaceutical products. By exploring these applications, we highlight the indispensable nature of high-quality scientific instruments in advancing laboratory practices.
Introduction
NIR infrared technology is revolutionizing pharmaceutical laboratories, delivering innovative solutions that significantly enhance efficiency, accuracy, and safety. As the industry increasingly embraces these advancements, it is crucial for professionals to understand the diverse applications of NIR infrared to optimize their processes effectively.
However, amid the promise of improved quality control and regulatory compliance, one must consider the challenges that may arise during the integration of this technology. A thorough exploration of the multifaceted uses of NIR infrared in pharmaceuticals not only highlights its transformative potential but also uncovers the complexities associated with its implementation.
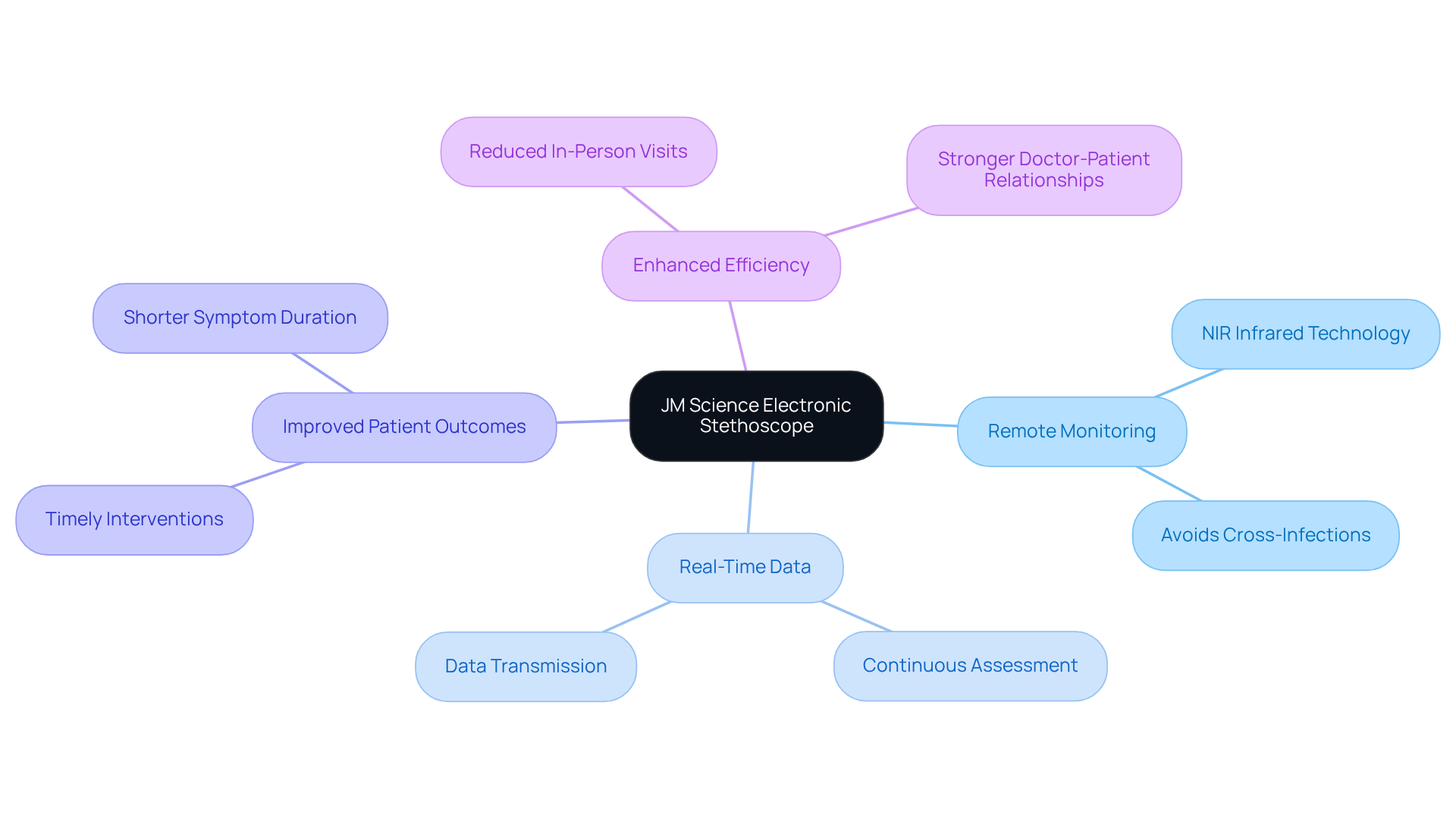
JM Science Electronic Stethoscope: Enhancing Remote Patient Monitoring with NIR Infrared
The JM Science Electronic Stethoscope revolutionizes remote patient monitoring by utilizing NIR infrared methods. This state-of-the-art device empowers healthcare professionals to from a distance, significantly enhancing accessibility and efficiency in patient care.
By leveraging NIR infrared technology, the stethoscope provides real-time data, facilitating timely interventions and reducing the need for in-person visits—an essential feature for effectively managing chronic conditions. Research indicates that remote monitoring tools like this can lead to improved patient outcomes, enabling continuous assessment and prompt responses to health changes.
Additionally, the integration of NIR infrared systems enhances the stethoscope's efficiency, ensuring high-quality sound capture even in challenging environments. Experts concur that the adoption of electronic stethoscopes with NIR infrared capabilities represents a substantial advancement in healthcare, bridging gaps in patient monitoring and fostering stronger doctor-patient relationships.
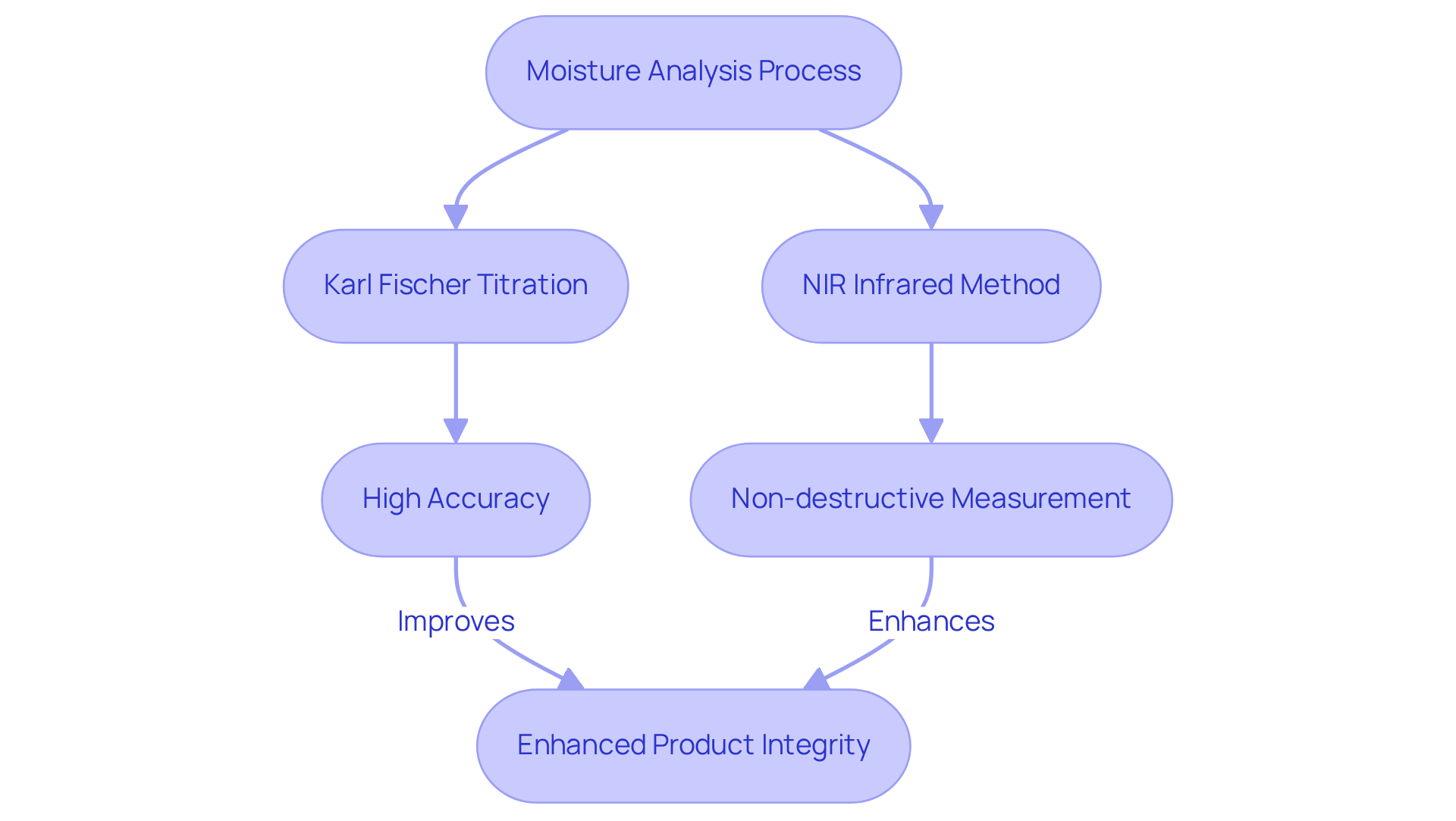
Karl Fischer Titrators: Utilizing NIR Infrared for Accurate Moisture Analysis
Karl Fischer titrators, notably the Hiranuma Aquacounter AQV-300 Volumetric and AQ-300 Coulometric models, are indispensable in moisture analysis within the pharmaceutical industry, particularly for compliance with the Japanese Pharmacopoeia. The integration of NIR infrared methods enhances accuracy and significantly boosts efficiency. NIR spectroscopy provides a non-destructive approach to moisture content measurement, allowing for rapid assessments that eliminate the need for extensive sample preparation. This technique can accurately in bulk powders within the range of 0.5-5% w/w, rendering it especially effective for pharmaceutical applications.
By employing NIR methods in conjunction with Karl Fischer titration, laboratories can streamline their processes, ensuring adherence to stringent regulatory standards while upholding high-quality control. The ability to consistently monitor moisture levels facilitates precise adjustments in production, ultimately elevating product standards and reducing waste. This synergy between Karl Fischer titration and NIR infrared technology exemplifies a progressive approach to moisture analysis in the drug industry, enhancing both operational efficiency and product integrity.
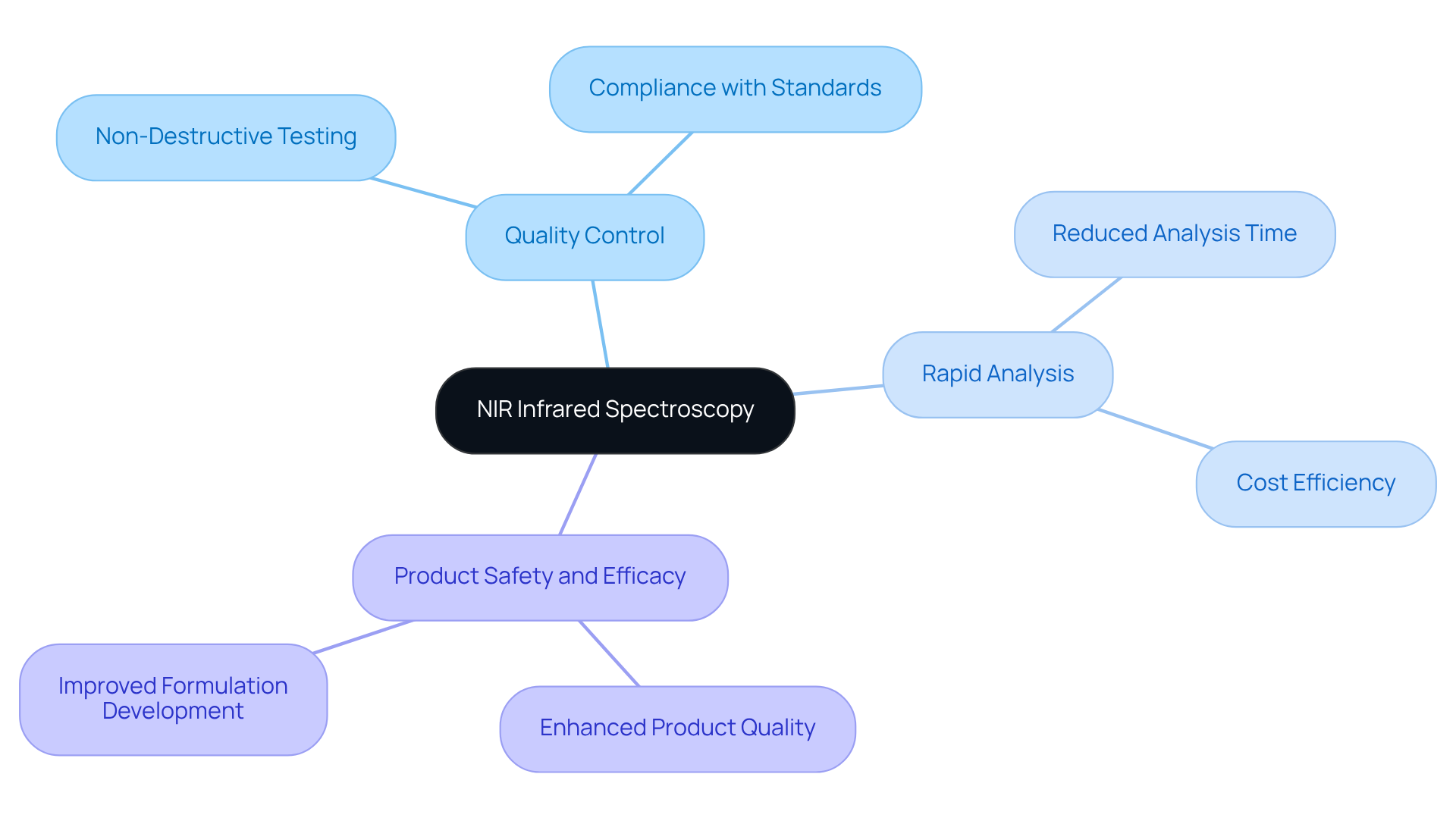
NIR Infrared Spectroscopy: Streamlining Quality Control in Pharmaceutical Laboratories
NIR infrared spectroscopy is a cornerstone in the field of standards management within drug laboratories. This innovative technique facilitates rapid and non-destructive analysis of both raw materials and finished products, ensuring compliance with predefined specifications. By integrating NIR infrared spectroscopy into their processes, laboratories can significantly streamline their quality control measures, thereby reducing analysis time. Ultimately, this advancement enhances overall , making it an indispensable tool in modern laboratory settings.
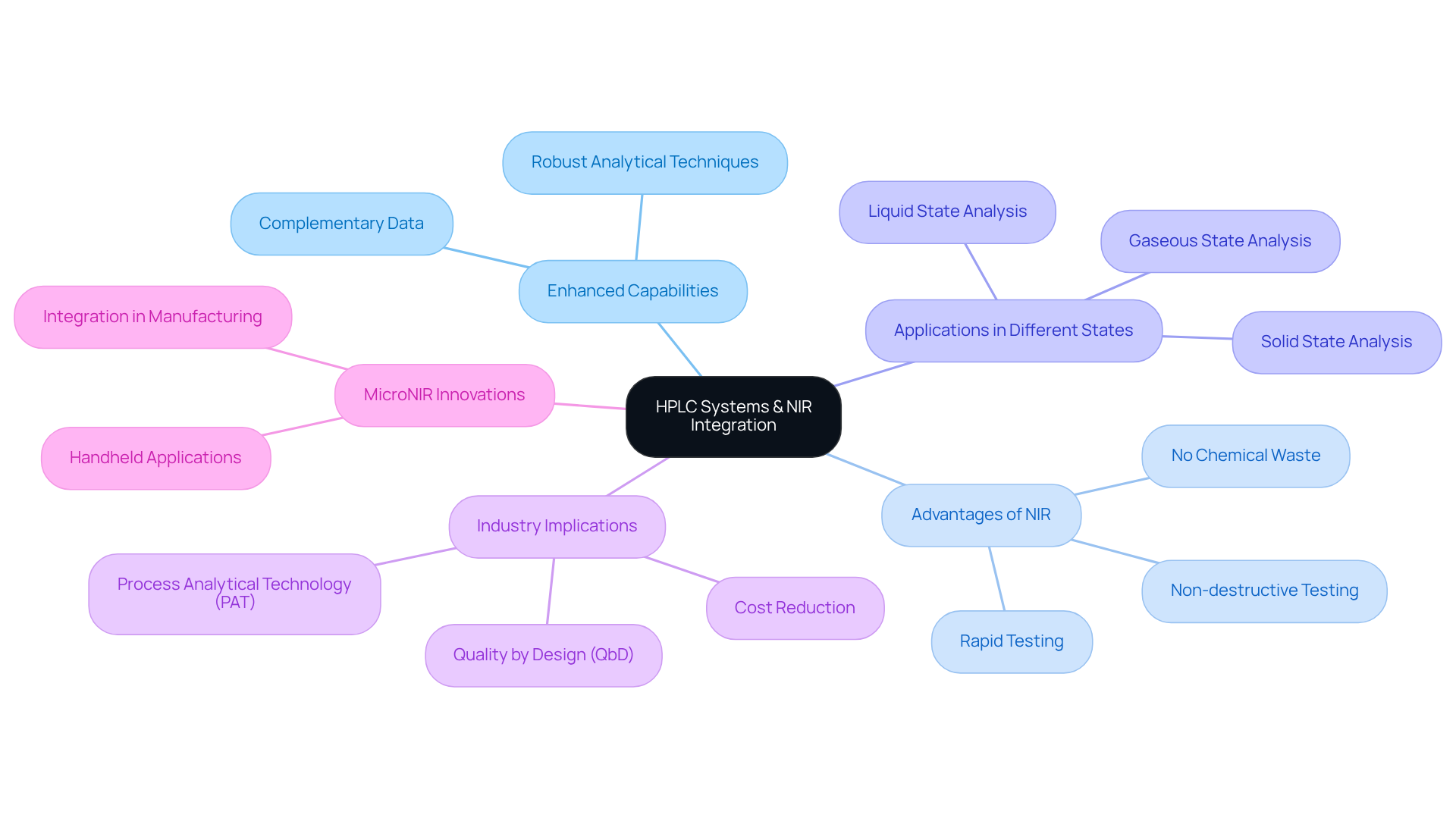
HPLC Systems: Leveraging NIR Infrared for Enhanced Pharmaceutical Analysis
High-Performance Liquid Chromatography (HPLC) systems are pivotal in drug analysis, and the integration of NIR infrared technology significantly enhances their capabilities. By employing NIR spectroscopy alongside HPLC, laboratories gain complementary data that deepens the understanding of compound interactions and stability. This integration fosters more , facilitating the development of safer and more effective medical products. Notably, NIR spectroscopy is non-destructive and non-invasive, enabling rapid testing without generating chemical waste, a critical advantage in quality control applications.
Moreover, the ability to analyze samples in solid, liquid, or gaseous states with equal accuracy positions NIR as an indispensable tool in the pharmaceutical industry. Consequently, the incorporation of NIR methods into HPLC workflows supports the industry's transition towards Quality by Design (QbD) and Process Analytical Technology (PAT), ultimately enhancing process control and reducing manufacturing costs. Furthermore, MicroNIR devices, designed for handheld operation, exemplify the practical applications of NIR innovations in drug manufacturing.
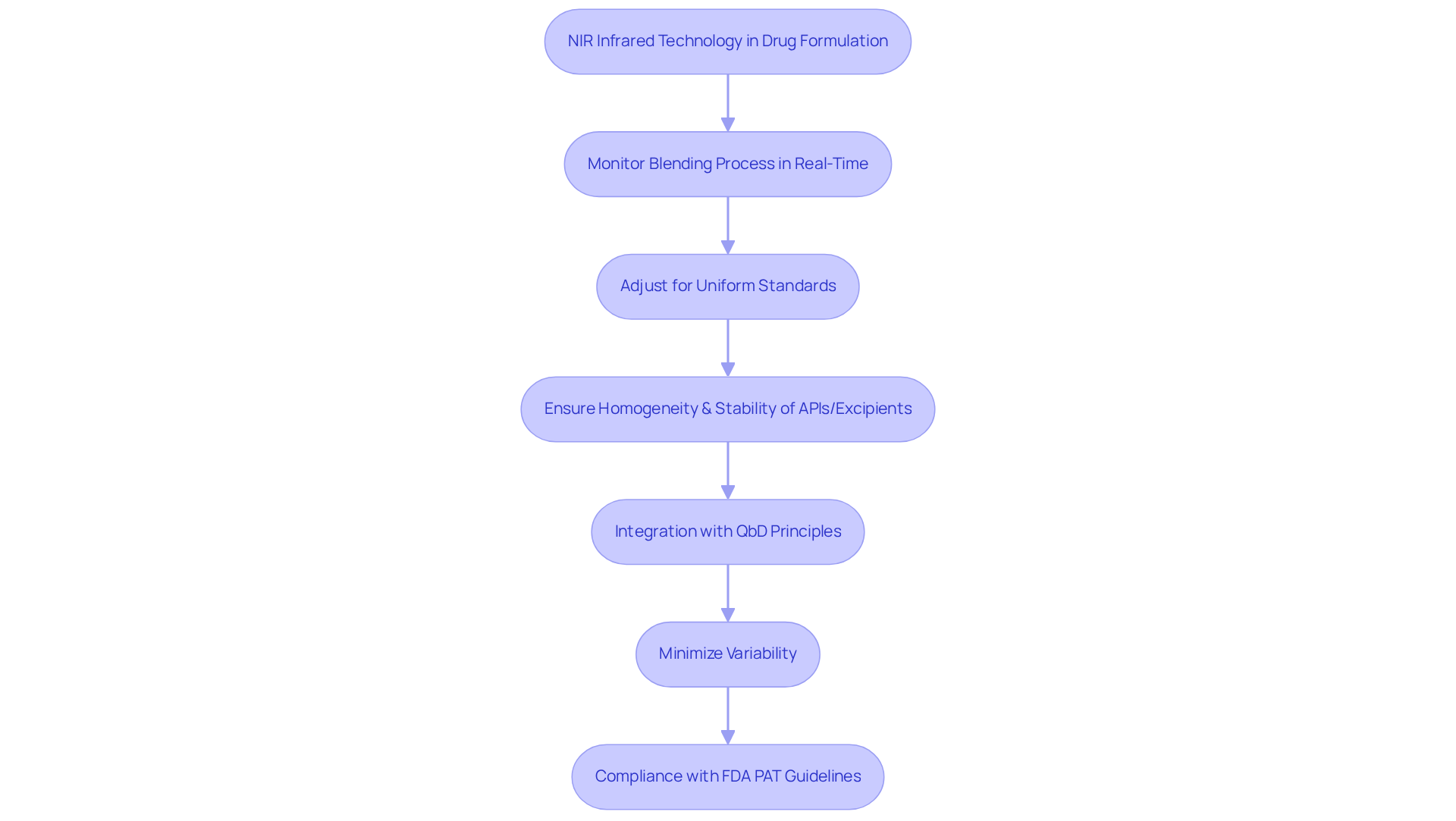
NIR Infrared in Drug Formulation Development: Ensuring Homogeneity and Stability
NIR infrared technology is essential in the development of drug formulations, ensuring the homogeneity and stability of active pharmaceutical ingredients (APIs) and excipients. Utilizing NIR spectroscopy, formulators can monitor the blending process in real-time, allowing for immediate adjustments to maintain uniform standards. This capability is crucial for developing formulations that not only meet regulatory standards but also provide reliable therapeutic outcomes.
Notably, studies have demonstrated that NIR can detect small API concentrations as low as 0.10 %w/w in powder blends, while also monitoring powder blends with concentrations as low as 0.5 %w/w. This underscores NIR's in maintaining uniformity.
Furthermore, the integration of NIR with Quality by Design (QbD) principles enables a systematic approach to method development, enhancing the understanding of critical quality attributes (CQAs) and minimizing variability. As Emil W. Ciurczak noted, the evolution of NIR from bulky laboratory instruments to compact, rugged tools has significantly improved its application in the industry.
Additionally, Alexander L. Bowler highlighted that measurement methods can enhance industrial mixing processes and elevate product standards by monitoring essential process parameters. Practical applications, such as the TANDEM online tablet characterization tool, demonstrate how NIR methods can guarantee standards by measuring tablet weight, thickness, hardness, and NIR content. This emphasizes the significance of uniformity in drug formulations.
The FDA's Process Analytical Technology (PAT) guidelines, released in 2004, further endorse the use of NIR in drug applications, highlighting its crucial role in improving control standards and adherence.
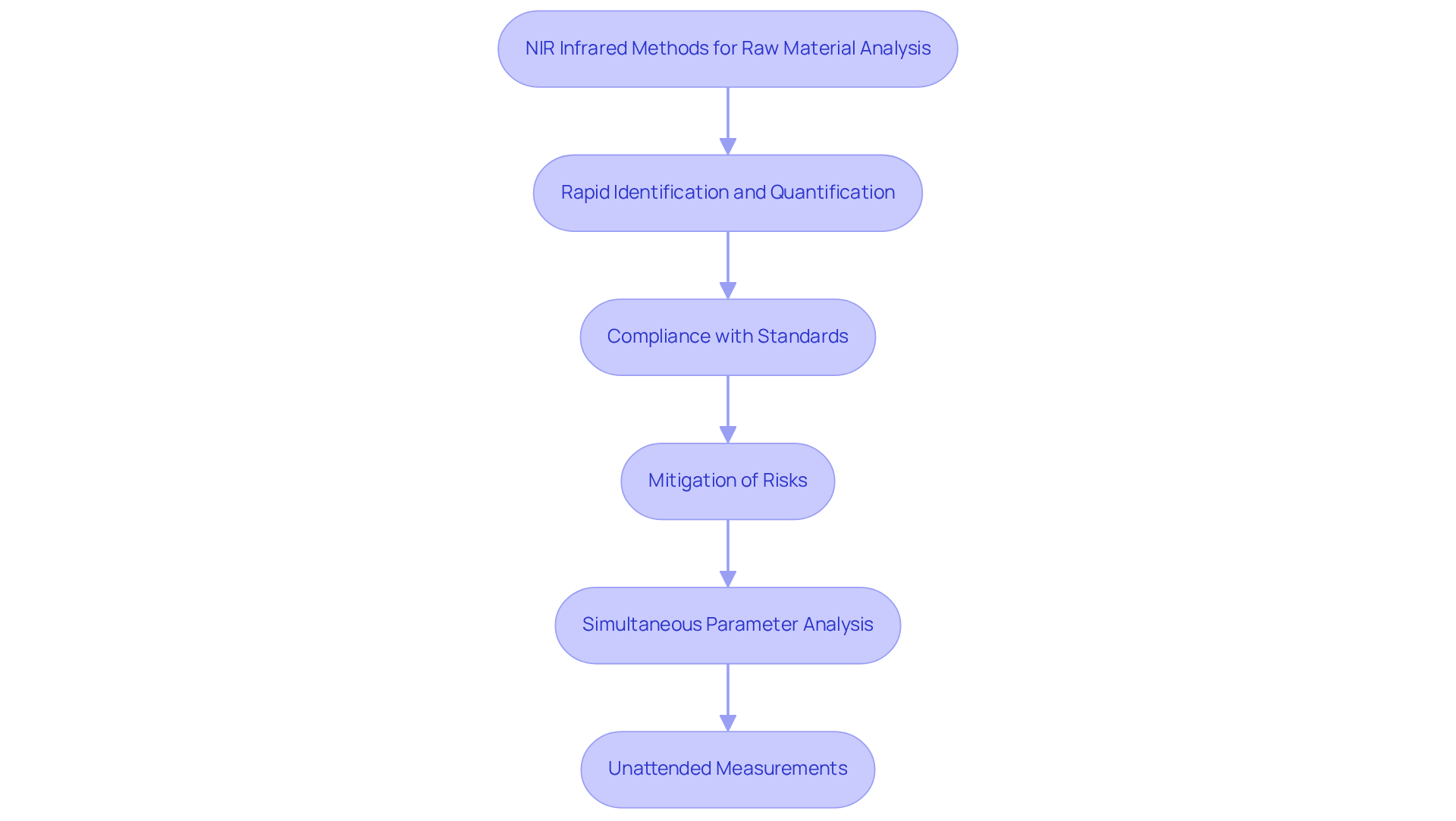
Raw Material Analysis: Implementing NIR Infrared for Quality Assurance
The application of NIR infrared methods for raw material analysis is crucial for ensuring compliance with standards in pharmaceutical manufacturing. NIR infrared spectroscopy enables the rapid identification and quantification of raw materials, facilitating swift adherence to required specifications before their use in production. This proactive approach significantly mitigates the risk of complications in the final product, thereby and effectiveness. Notably, NIRS can analyze multiple parameters simultaneously, delivering results in under one minute without necessitating sample preparation. Such efficiency is vital, as good manufacturing practices require testing 100% of raw materials. Furthermore, the ability to conduct unattended measurements of up to 30 tablets at once exemplifies the system's practicality in high-throughput environments. As highlighted in various pharmacopoeias, including European and American standards, the NIR infrared method is recognized as a reliable approach for maintaining stringent control within the pharmaceutical industry.
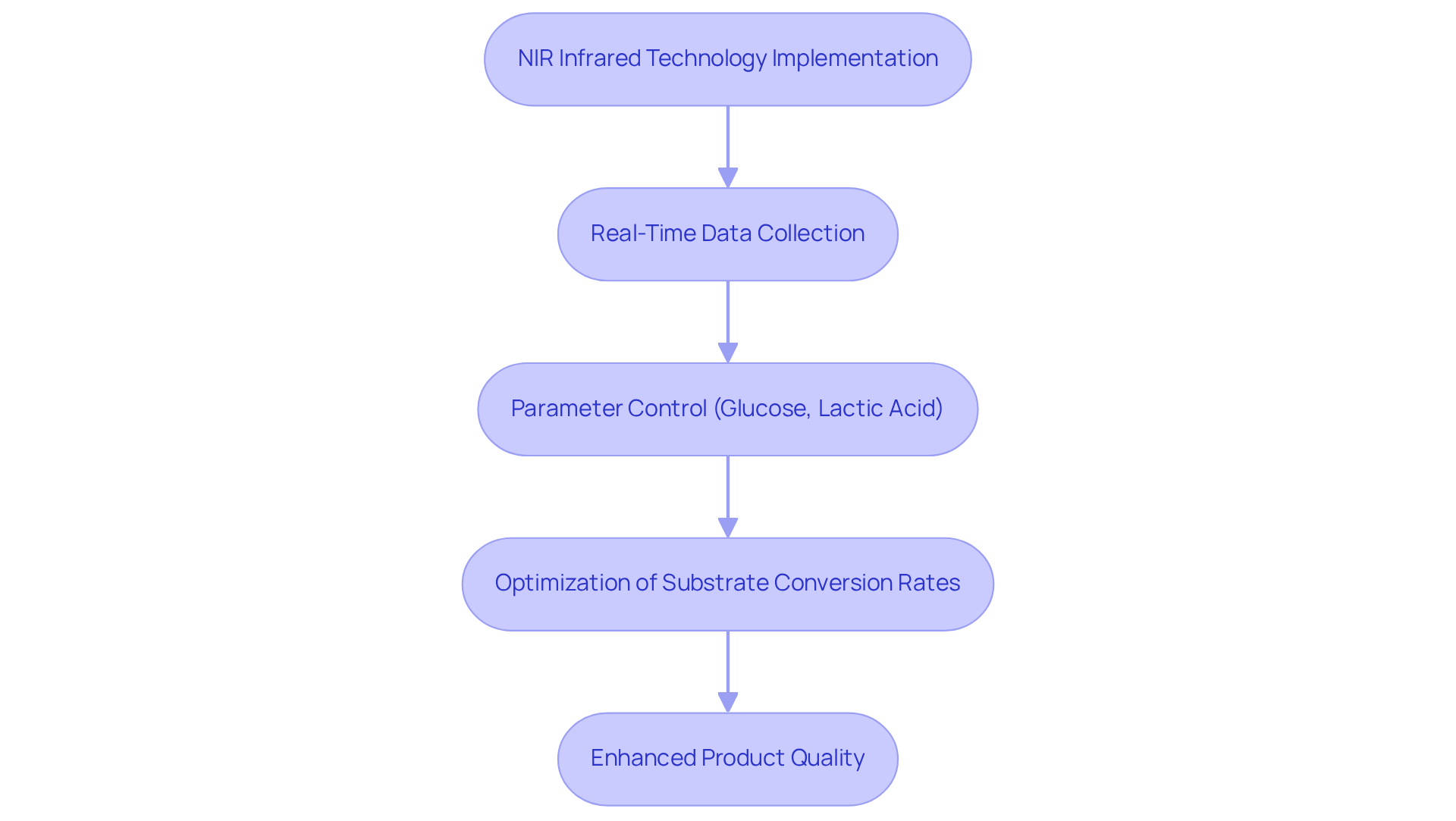
Chemical Reaction Monitoring: Utilizing NIR Infrared for Process Optimization
The monitoring of chemical reactions in pharmaceutical manufacturing is being revolutionized by . By delivering real-time data on reaction progress, NIR spectroscopy empowers chemists to fine-tune conditions for optimal yield and efficiency. This capability not only enhances productivity but also reduces waste, ensuring that final products conform to strict standards.
For instance, studies have demonstrated that the integration of NIR systems in fermentation processes allows for precise control of key parameters such as glucose and lactic acid concentrations, significantly enhancing process efficiency. Furthermore, the implementation of automated NIR monitoring has been shown to optimize substrate conversion rates, leading to more consistent product quality.
As noted by Elena Tamburini, 'Real-time nir infrared measurements of biochemical parameters have been shown to be a valuable tool for technical operators and profile controls.' The capability to perform real-time analysis is essential for upholding high standards in drug production, ultimately aiding in enhanced consumer satisfaction and regulatory compliance.
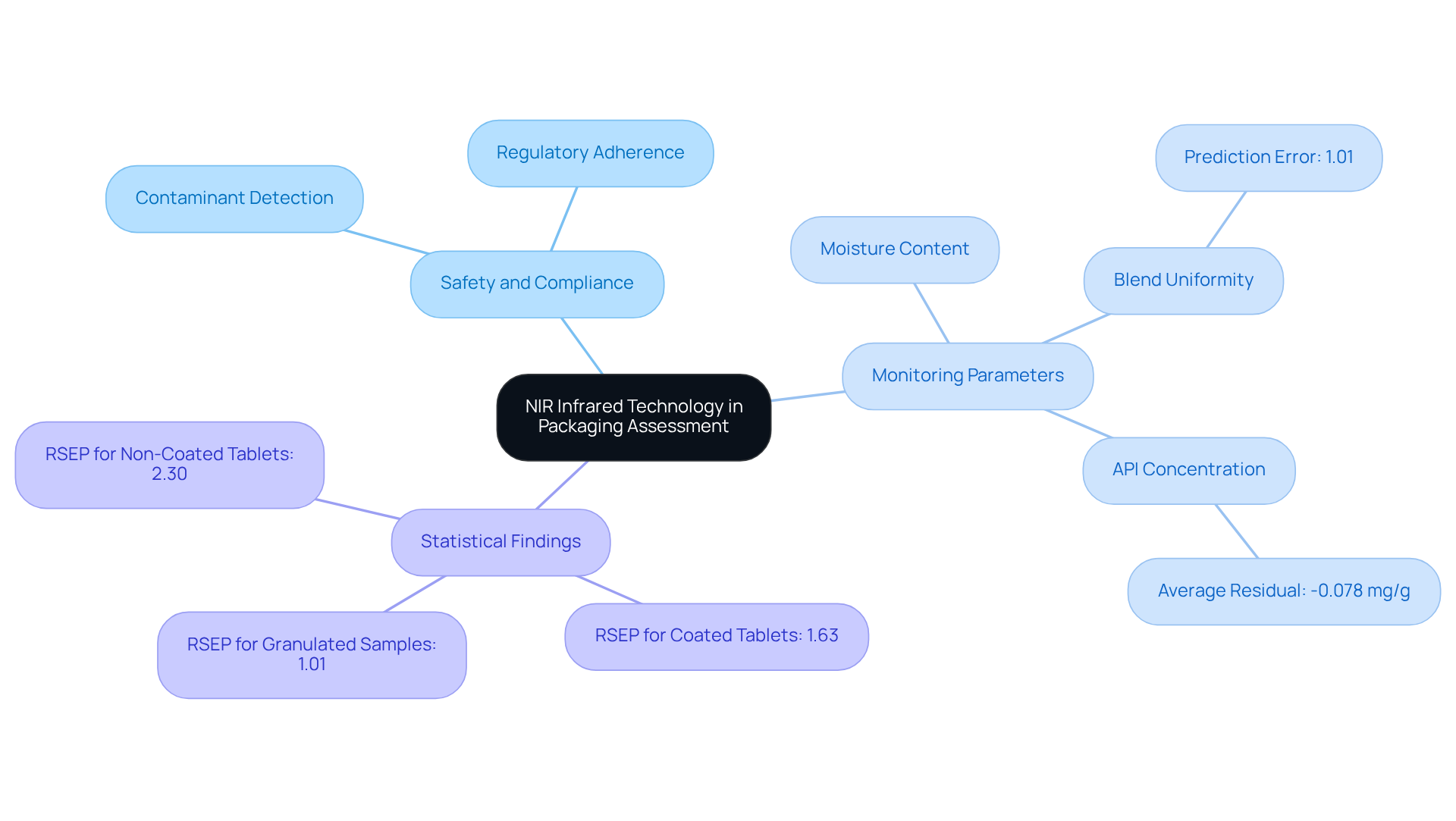
Packaging Material Assessment: NIR Infrared for Compliance and Safety
NIR infrared technology plays a crucial role in assessing packaging materials for safety and compliance in the healthcare sector. By analyzing the spectral properties of these materials, NIR infrared spectroscopy effectively detects potential contaminants and confirms adherence to regulatory standards. This capability is vital for maintaining the throughout their shelf life.
For example, research has shown that NIR spectroscopy can monitor blend uniformity and moisture content in packaging materials, ensuring they meet rigorous safety requirements. The prediction error for granulated samples was found to be 1.01%, while for tablets, it was 1.63%, underscoring the precision of NIR infrared technology in monitoring.
Furthermore, this method's application in practical scenarios, such as assessing active medicinal components (APIs) in coated tablets, validates its effectiveness in sustaining quality control, with an average residual for API concentration recorded at -0.078 mg/g. The capacity to detect variations in packaging materials not only enhances product safety but also facilitates compliance with industry regulations, ultimately protecting consumer health.
As M Blanco from the Universitat Autònoma de Barcelona noted, 'The results demonstrate that NIR infrared spectroscopy is a viable option compared to other, more time-consuming methods of analysis for monitoring processes in the drug industry.' Additionally, a case study confirming NIR infrared methods for API quantification highlighted its reliability for routine analysis, further emphasizing the significance of this innovation in the drug industry.
Stability Testing: Applying NIR Infrared to Determine Shelf Life and Efficacy
Employing nir infrared technology in stability testing is crucial for companies in the drug industry, as it enables them to effectively assess the shelf life and efficacy of their products. NIR spectroscopy provides a non-destructive method for analyzing chemical changes over time, allowing researchers to evaluate the stability of formulations under various conditions. This capability is essential for ensuring that products remain safe and effective throughout their intended .
For instance, research has demonstrated that nir infrared spectroscopy can precisely evaluate the stability of active drug ingredients (APIs) in formulations, providing vital information that guides shelf life assessments. Notably, NIRS can identify five active ingredients (APIs) in flu symptom relief tablets, showcasing its effectiveness in monitoring stability.
Furthermore, the efficacy of nir infrared methods has been validated for monitoring the degradation of compounds, ensuring compliance with regulatory standards. As the FDA emphasizes Quality by Design (QbD) and Process Analytical Technology (PAT), utilizing NIR spectroscopy empowers laboratories to streamline their stability testing processes, ultimately enhancing product safety and reliability.
However, it is important to consider the challenges faced in stability testing, such as the costs associated with impurity testing, which can impact the overall efficiency of the process.
Environmental Monitoring: NIR Infrared for Compliance in Pharmaceutical Laboratories
Innovations in NIR infrared technology are revolutionizing environmental oversight in drug laboratories, playing a pivotal role in ensuring compliance with stringent regulatory standards. By analyzing air and surface samples, NIR spectroscopy effectively detects contaminants and assesses the overall cleanliness of the laboratory environment. This capability is vital for maintaining a safe workspace, as studies show that can significantly mitigate contamination risks, thereby enhancing the quality of pharmaceutical products.
For example, NIR infrared spectroscopy facilitates rapid, non-destructive analysis, enabling laboratories to identify potential contaminants in real-time, which is essential for adhering to Good Manufacturing Practices (GMP). Moreover, the integration of NIR infrared technology promotes continuous monitoring, ensuring that laboratories remain compliant with industry regulations while optimizing operational efficiency.
Conclusion
NIR infrared technology is revolutionizing pharmaceutical laboratories by delivering innovative solutions that enhance quality control, streamline processes, and ensure compliance with regulatory standards. The applications highlighted demonstrate how NIR infrared methods are not only improving the efficiency of various laboratory practices but also playing a critical role in patient safety and product integrity.
From remote patient monitoring with electronic stethoscopes to moisture analysis using Karl Fischer titrators, NIR infrared technology enables rapid, non-destructive testing that meets the stringent demands of the pharmaceutical industry. The integration of NIR spectroscopy in drug formulation development and stability testing further underscores its importance in maintaining the efficacy and shelf life of pharmaceutical products. Additionally, its role in raw material analysis and environmental monitoring emphasizes the comprehensive benefits NIR infrared brings to pharmaceutical manufacturing processes.
As the pharmaceutical industry continues to evolve, embracing NIR infrared technology will be crucial for laboratories aiming to enhance operational efficiency and uphold the highest standards of quality. By investing in these advanced methods, stakeholders can ensure a safer and more effective healthcare environment, ultimately benefiting both manufacturers and patients alike.




