Overview
This article presents ten essential insights regarding ASTM D664, underscoring its critical role for pharmaceutical lab managers in achieving precise acid number testing for quality control. By adhering to ASTM D664 standards, laboratories can significantly enhance measurement precision, reduce variability, and ensure compliance with regulatory mandates. Such adherence is vital for preserving product integrity and optimizing operational efficiency within pharmaceutical environments. Ultimately, these insights reinforce the importance of high-quality scientific instruments in laboratory settings.
Introduction
The pharmaceutical industry is fundamentally reliant on stringent quality control measures to uphold product integrity and safety. Among these essential protocols, ASTM D664 emerges as a pivotal standard for evaluating the acidity of various substances, including oils and lubricants. This article presents ten key insights that pharmaceutical lab managers can harness to refine their testing processes and ensure compliance with industry regulations. As advancements in technology and methodologies continue to progress, the pressing question remains: how can laboratories effectively integrate these insights to navigate challenges and optimize operational efficiency?
JM Science Inc.: Advanced Titrators for ASTM D664 Acid Number Testing
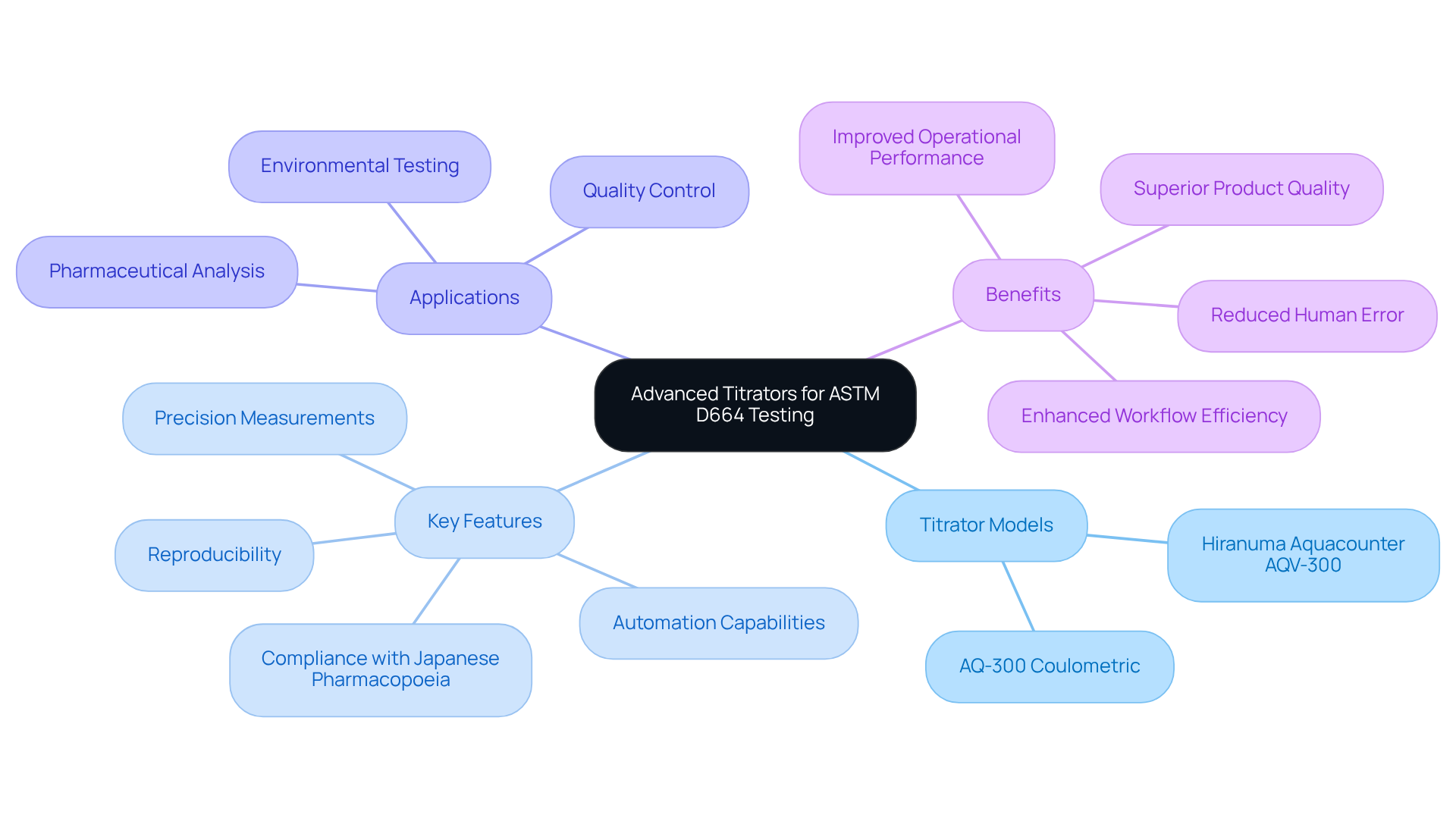
JM Science Inc. offers a comprehensive selection of sophisticated titrators tailored for evaluating the ASTM D664 acid number, with a particular focus on potentiometric titrators that excel within the pharmaceutical industry. Notably, the Hiranuma Aquacounter AQV-300 Volumetric and AQ-300 Coulometric Karl Fischer Titrators are meticulously designed for drug and medicine analysis, ensuring adherence to the Japanese Pharmacopoeia. These advanced instruments are equipped with features that guarantee precise measurements and reproducibility—elements that are vital for maintaining high-quality standards in pharmaceutical environments.
The automation capabilities of these titrators dramatically reduce human error and enhance workflow efficiency, rendering them essential tools for pharmaceutical lab managers who require dependable results in their quality control processes. By integrating these advanced titration systems, laboratories can streamline their testing procedures while ensuring compliance with rigorous industry standards. This ultimately leads to and superior product quality, solidifying the laboratory's commitment to excellence in pharmaceutical analysis.
ASTM D664 Standard: Overview and Importance in Acid Number Testing
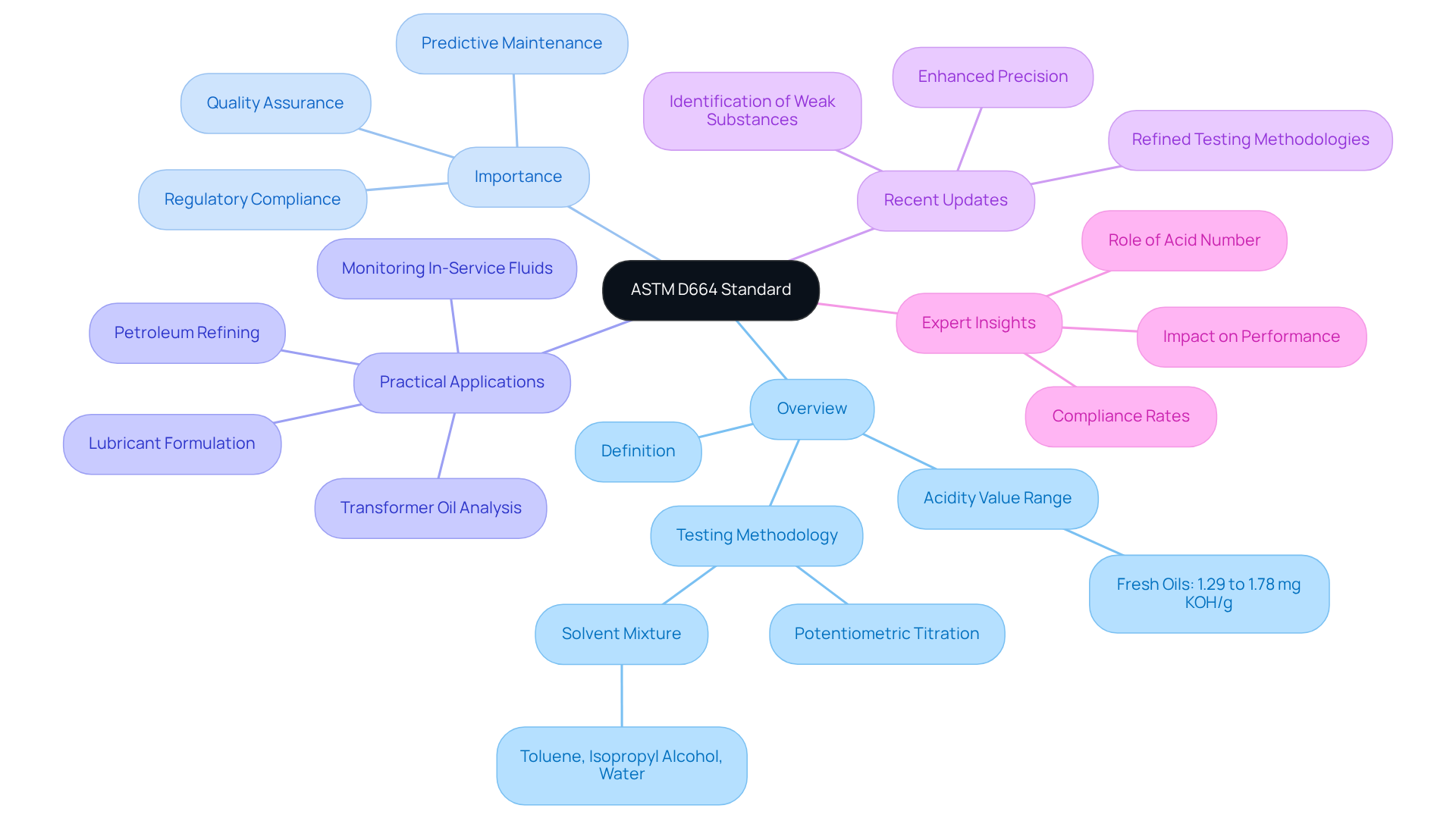
ASTM D664 is the definitive standard testing procedure for evaluating the acidity value of petroleum products, lubricants, and biodiesel through potentiometric titration. This standard is essential for assessing the quality and stability of these products, as the reveals the presence of sour components that can significantly impact performance and durability. For instance, the total acidity value (TAN) for fresh oils typically ranges from 1.29 to 1.78 mg KOH/g, providing a reliable benchmark for product integrity evaluation. By adhering to ASTM D664, laboratories can ensure trustworthy and consistent assessment methods, which are vital for maintaining product integrity and meeting regulatory standards.
The practical applications of ASTM D664 are extensive, particularly within industries such as petroleum refining, lubricant formulation, and transformer oil analysis. These sectors rely on precise acidity data to monitor in-service fluids and guarantee optimal performance. Recent updates to ASTM D664 have refined testing methodologies, enhancing the precision and efficiency of acidity evaluations. Notable improvements include modifications to the titration procedure that facilitate better identification of weak substances.
Expert insights emphasize the importance of the acid number in petroleum products, underscoring its role in predicting potential degradation and identifying concerns related to oxidation and additive breakdown. Current compliance rates with ASTM D664 in testing facilities are critical, as they reflect the industry's dedication to quality assurance and regulatory adherence. It is imperative for facilities to employ consistent assessment techniques and maintain uniform evaluation environments to ensure reliable outcomes. By implementing ASTM D664, laboratories not only enhance the overall reliability of petroleum products across various applications but also uphold high testing standards.
Methodology of ASTM D664: Step-by-Step Process for Acid Number Determination
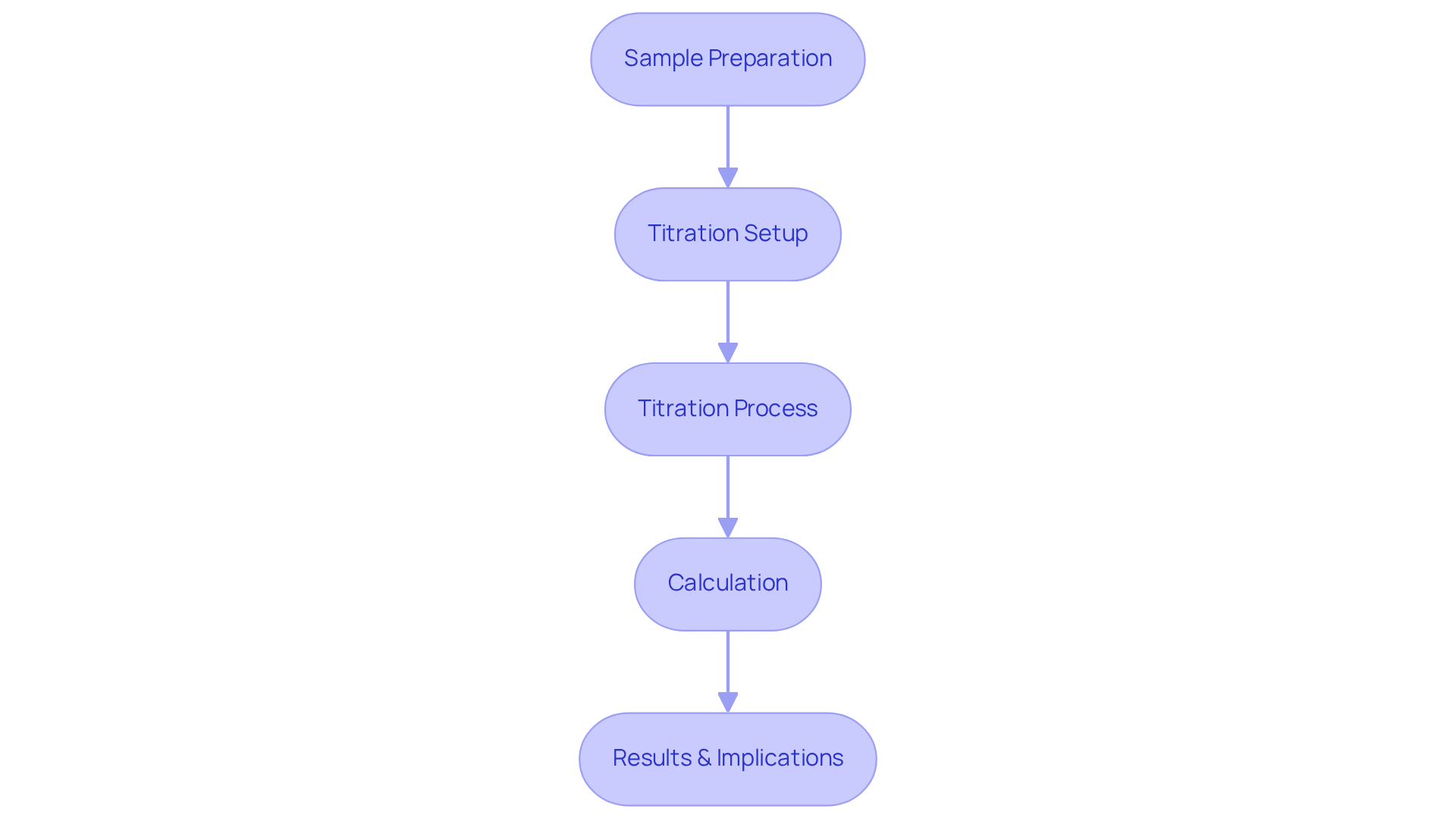
The for determining the acid number includes several critical steps to ensure accuracy and compliance.
- Sample Preparation: Begin by weighing a precise amount of the sample and dissolving it in an appropriate solvent, typically a mixture of toluene and isopropanol. This step is crucial for achieving a homogenous solution that facilitates accurate titration.
- Titration Setup: Prepare the titration apparatus, ensuring that the pH electrode is properly calibrated. Calibration is essential for dependable endpoint detection; discrepancies can lead to substantial errors in acidity calculations.
- Titration Process: Gradually add a standardized potassium hydroxide (KOH) solution to the sample while continuously stirring. Monitor the pH closely until the endpoint is reached, indicated by a stable pH reading. This process requires careful attention, as over-titration can skew results.
- Calculation: Finally, calculate the acid number based on the volume of KOH used during the titration. Accurate calculations are vital, reflecting the oil's acidity and indicating potential issues such as additive depletion or oxidation.
Adhering meticulously to these steps ensures accurate results and aligns with best practices in laboratory settings. For instance, studies have demonstrated that following the ASTM D664 method can yield a repeatability of +/- 7 percent for fresh oils, as well as a reproducibility of +/- 20 percent for fresh oils and +/- 44 percent for used oils, underscoring the reliability of ASTM D664. Furthermore, integrating systematic testing protocols based on equipment criticality can enhance maintenance strategies, as evidenced by CP Kelco's significant cost savings through comprehensive condition monitoring. By implementing these best practices, pharmaceutical labs can effectively manage oil quality and maintain equipment reliability. Elevated total acidity values can lead to bearing corrosion and seal degradation, highlighting the significance of tracking Total Acid Number (TAN) for equipment well-being.
Understanding Acid Number Limits: Implications for Quality Control
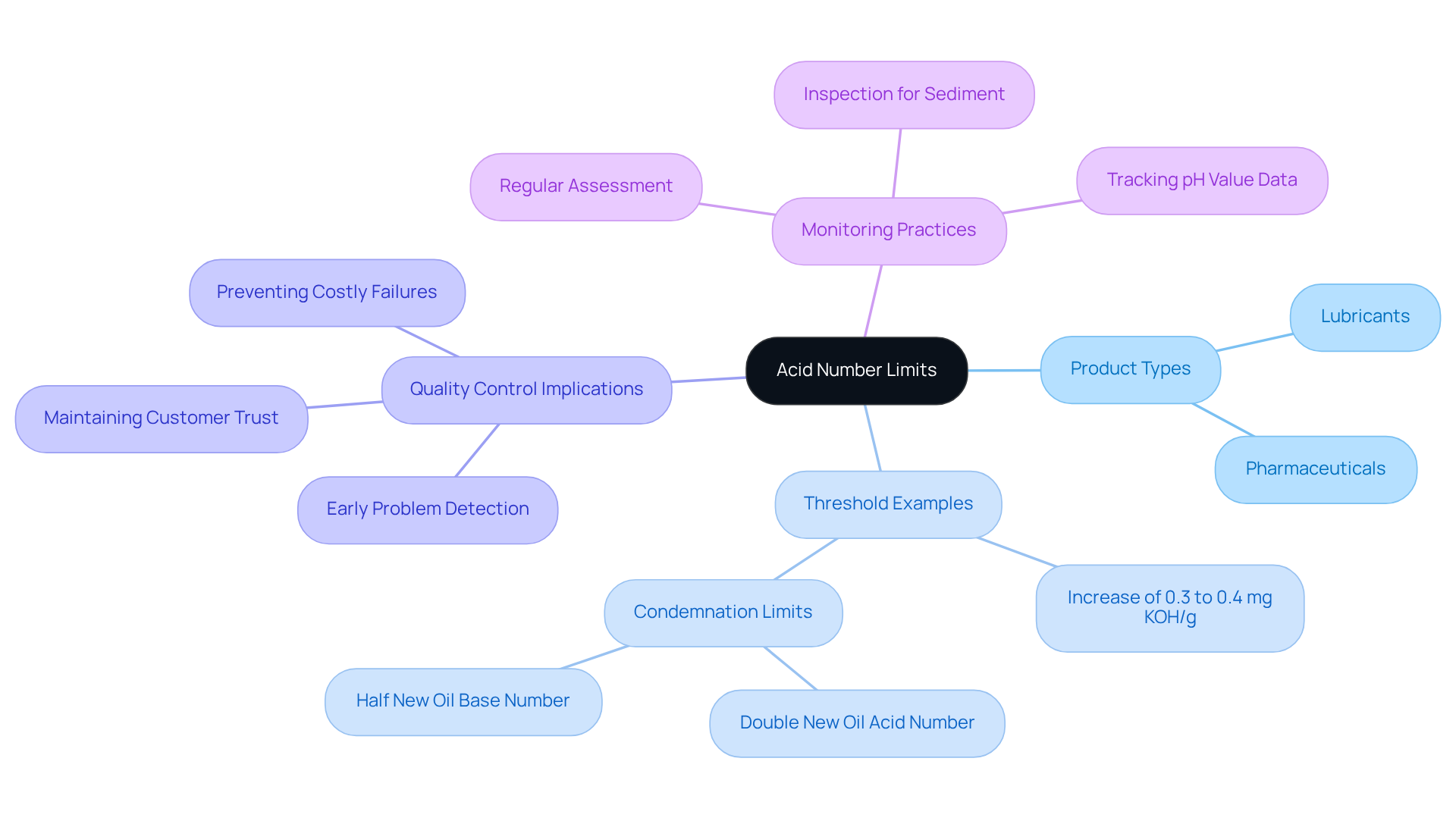
Efficient quality management in laboratories hinges on a comprehensive understanding of pH limits. These limits are not uniform; they vary by product type, with lubricants often exhibiting specific thresholds that indicate potential degradation or contamination when surpassed.
For example, an increase of 0.3 to 0.4 mg KOH/g above the initial value typically signals that the oil is nearing the end of its service life. This necessitates immediate examination for sediment in filters and centrifuges.
Consistent tracking of pH value data against established thresholds is crucial for early problem detection, thereby ensuring that products meet safety and performance criteria. Statistics indicate that oil should be condemned at , emphasizing the critical importance of monitoring these limits set by ASTM D664.
Furthermore, a significant proportion of product failures can be attributed to pH levels exceeding acceptable limits, emphasizing the necessity for diligent oversight. By implementing robust monitoring practices, managers can not only avert costly failures but also maintain customer trust and satisfaction.
As industry specialists assert, regular assessment of acidity limits is essential for preserving product quality and enhancing operational effectiveness in pharmaceutical applications.
Ensuring Reproducibility: Best Practices for Accurate Acid Number Testing
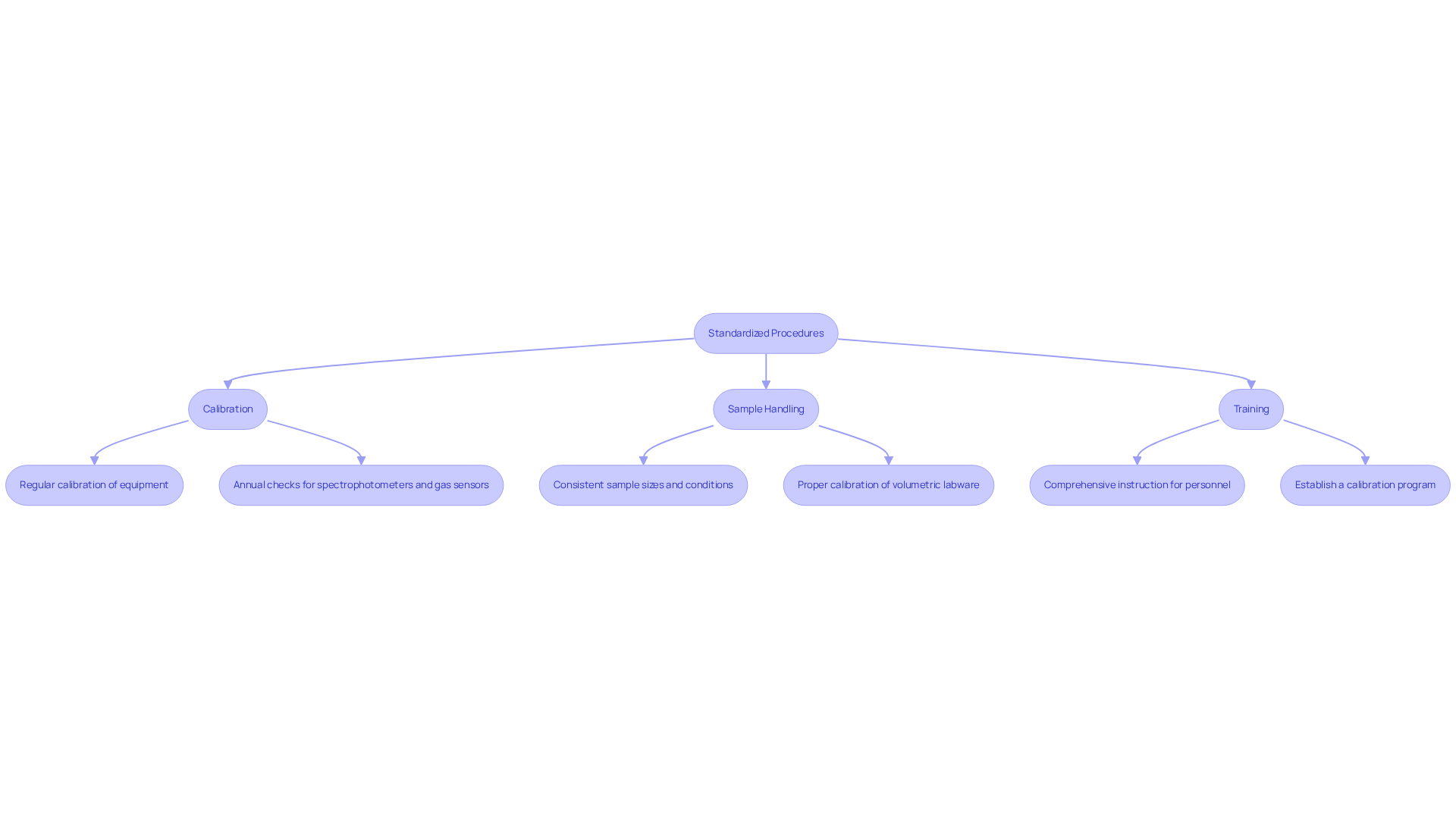
To ensure , facilities must adopt several best practices. Standardized procedures are paramount; adhering strictly to ASTM D664 enhances the reliability of results and minimizes variability.
- Calibration is equally crucial. Regular calibration of titration equipment and pH electrodes is essential, with spectrophotometers and gas sensors requiring annual checks to maintain accuracy. As noted, 'spectrophotometers, gas sensors, orifice plates, pitot tubes, gas meters, and rotor meters must be calibrated annually by an accredited facility.'
- Sample Handling is another critical aspect. Consistent sample sizes and conditions are vital to reduce variability in results, ensuring that measurements remain comparable. Proper calibration of volumetric labware, such as flasks and pipettes, is also necessary to maintain measurement integrity.
- Training is indispensable; comprehensive instruction for all personnel in evaluation procedures minimizes human error and upholds quality standards. Connie Shek emphasizes that 'if you work to ISO 9001:2015 and/or ISO 17025:2017 standards, it’s imperative that you establish a calibration programme that ensures the validity of the results given by your weighing equipment.'
By adopting these practices, facilities can achieve consistent and dependable acidity assessments, essential for quality assurance and adherence to industry standards. Regularly reviewing and adjusting your calibration schedule based on equipment usage and manufacturer recommendations ensures optimal performance.
Standardized Methods: Key to Reliable Acid Number Testing
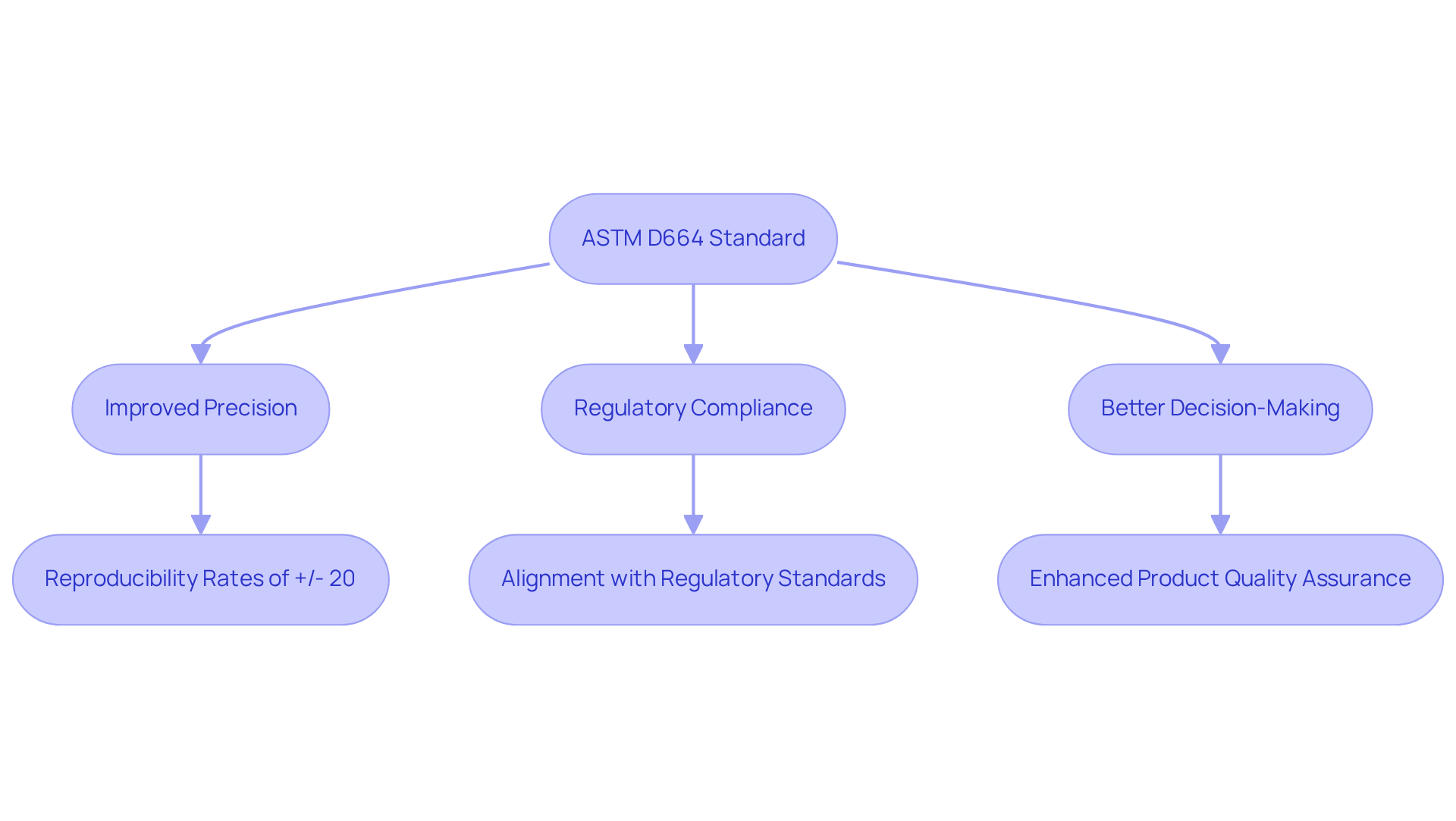
Standardized methods, particularly ASTM D664, are essential for achieving reliable acid number testing in laboratory settings. By providing a consistent framework, ASTM D664 minimizes variability in results, allowing testing centers to effectively compare findings across different tests and facilities. This standardization not only bolsters the credibility of testing results but also ensures alignment with increasingly critical in the pharmaceutical sector.
Facilities that have implemented the ASTM D664 have reported improved precision and consistency in their testing processes, achieving reproducibility rates of +/- 20 percent for fresh oils. The acid number itself is defined as the mass (in mg) of potassium hydroxide required to neutralize 1 g of a sample, which is vital for contextualizing the measurement.
Experts emphasize that adherence to such standardized methods is not merely a best practice; it is a necessity for facilities striving to meet stringent regulatory standards. By adopting the ASTM D664 standard, laboratories can guarantee that their testing results are both dependable and compliant, ultimately facilitating better decision-making in product development and quality assurance.
Furthermore, monitoring the acid number in in-service lubricants is crucial for detecting oil degradation and oxidation, highlighting the significance of ASTM D664 in quality control across various sectors, including crude oils, refined products, and lubricants.
Total Acid Number vs. Acid Number: Understanding the Differences
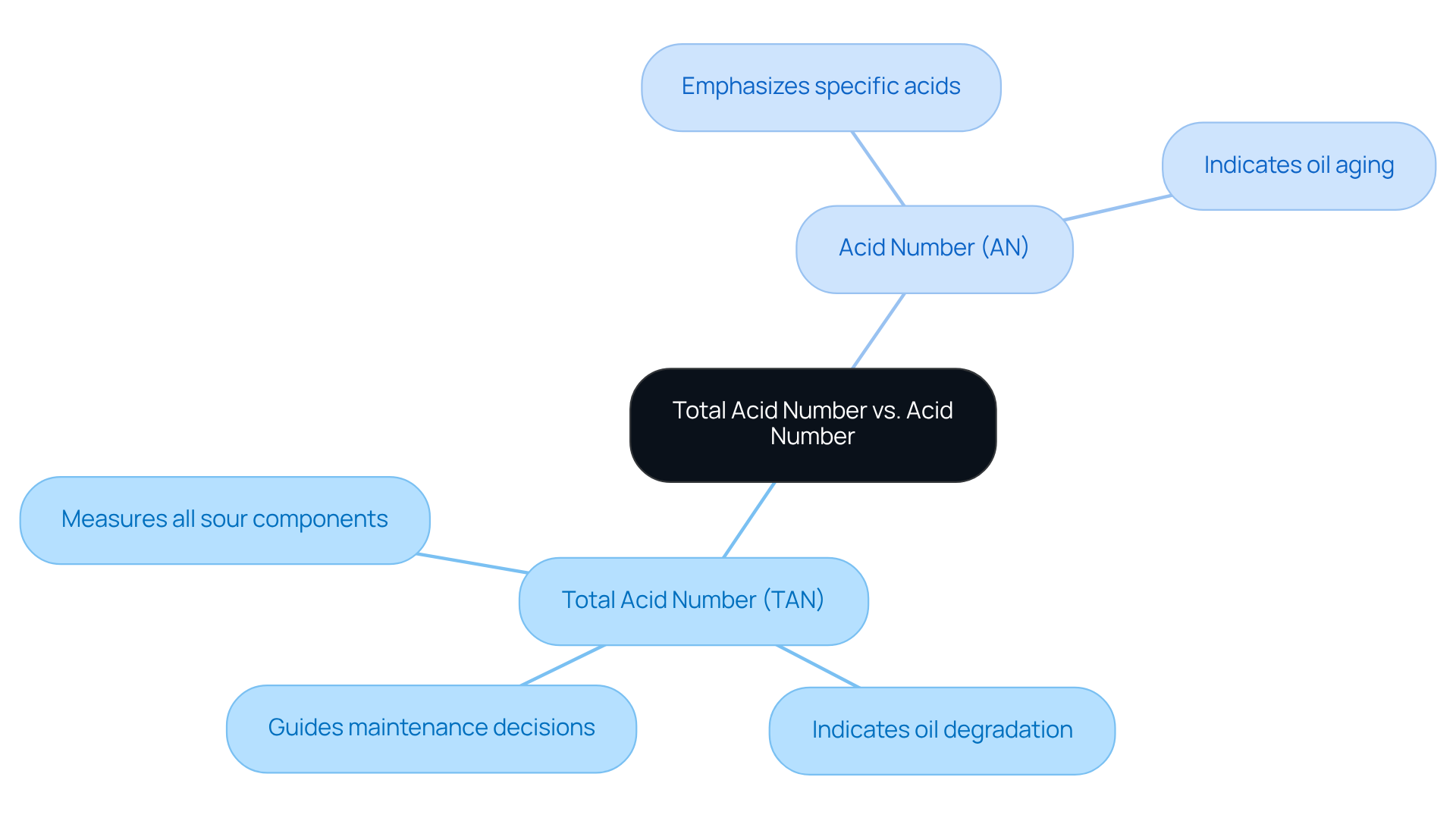
The terms Total Acid Number (TAN) and Acid Number (AN) are often confused, yet they represent distinct measurements that are critical to oil analysis. TAN measures all sour components in a sample, providing a comprehensive perspective on oil quality, while AN typically emphasizes the levels of specific acids. This distinction is vital; misinterpretation can lead to erroneous product evaluations and .
For instance, a significant increase in TAN may indicate oil degradation, potentially resulting in premature wear on equipment if not addressed promptly. High TAN values can also lead to seal deterioration and fluid leaks, underscoring the practical consequences of misunderstanding these measurements.
Therefore, laboratory managers must ensure their teams grasp these differences to avoid costly mistakes in analysis. Regular monitoring of TAN is essential, as it reflects the overall condition of lubricants and can effectively guide maintenance decisions, in accordance with ASTM D664. Monitoring TAN aids in optimizing maintenance by timing oil changes appropriately.
As noted by Rich Wurzbach, an expert in the field, the Acid Number of a lubricant indicates oil aging and degradation, highlighting the importance of integrating TAN insights with other oil analysis parameters into routine laboratory practices.
pH Influence: How It Affects Acid Number Measurements
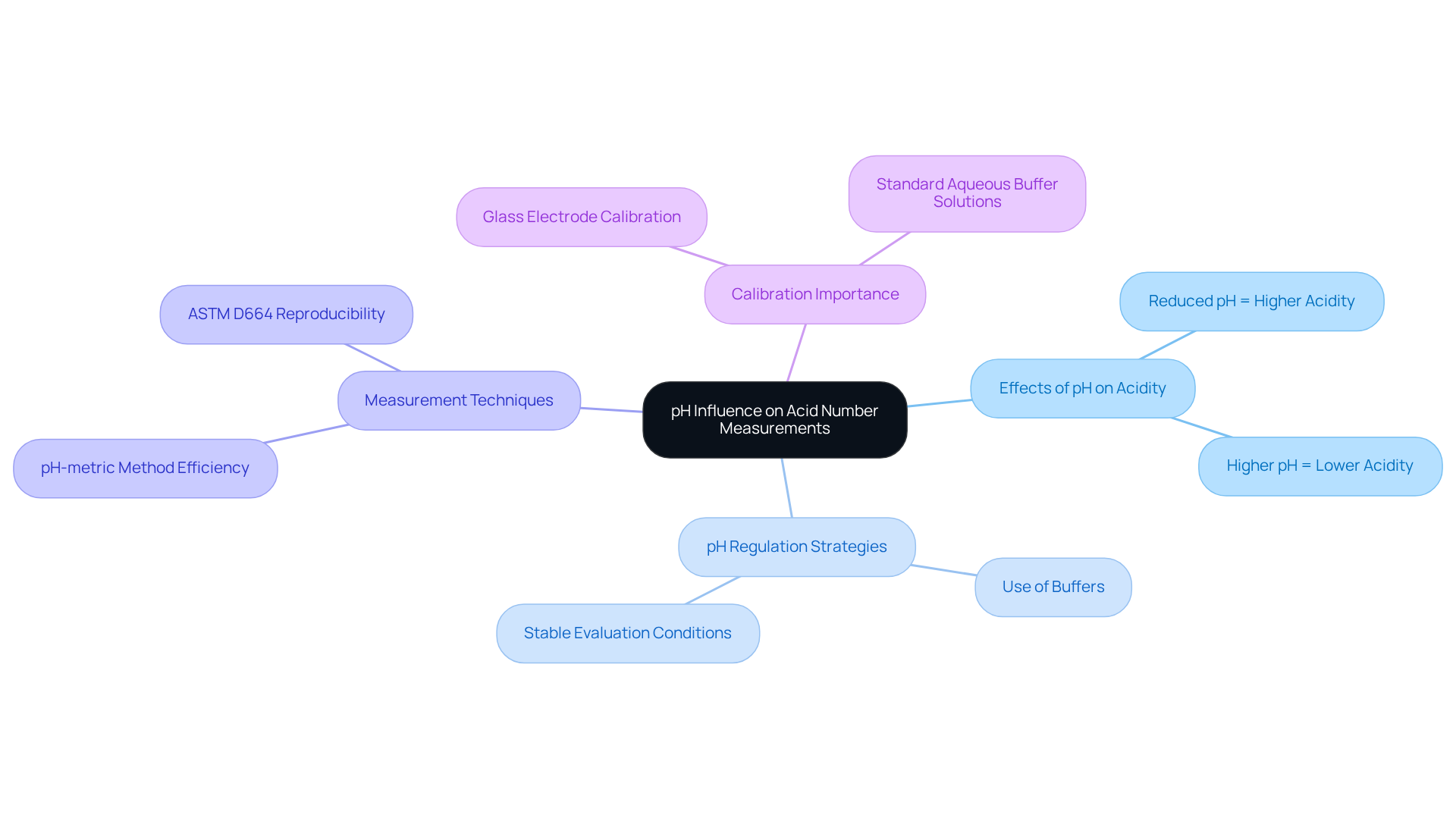
The pH of a sample significantly influences acidity measurements. A reduced pH indicates a higher concentration of sour substances, leading to increased acidity number measurements. Conversely, a higher pH suggests a lower concentration of these substances. Therefore, laboratory managers must diligently during evaluations to guarantee accurate results.
Implementing pH regulation strategies, such as utilizing appropriate buffers and ensuring stable evaluation conditions, is essential in mitigating the impact of pH variations on acidity assessments. Notably, the pH-metric method is efficient, yielding responses in just one minute, which underscores the effectiveness of automated pH monitoring systems.
Furthermore, pH measurements are conducted with a glass electrode calibrated using standard aqueous buffer solutions, highlighting the critical role of proper calibration in achieving reliable outcomes. Additionally, the incorporation of spiking agents during acidity titration has demonstrated enhanced precision, exemplifying a practical application of pH control measures.
It is important to recognize that ASTM D664 indicates a reproducibility of +/- 20 percent of the mean for fresh oils 95 percent of the time, emphasizing the necessity of maintaining stable pH conditions to minimize variability in test results.
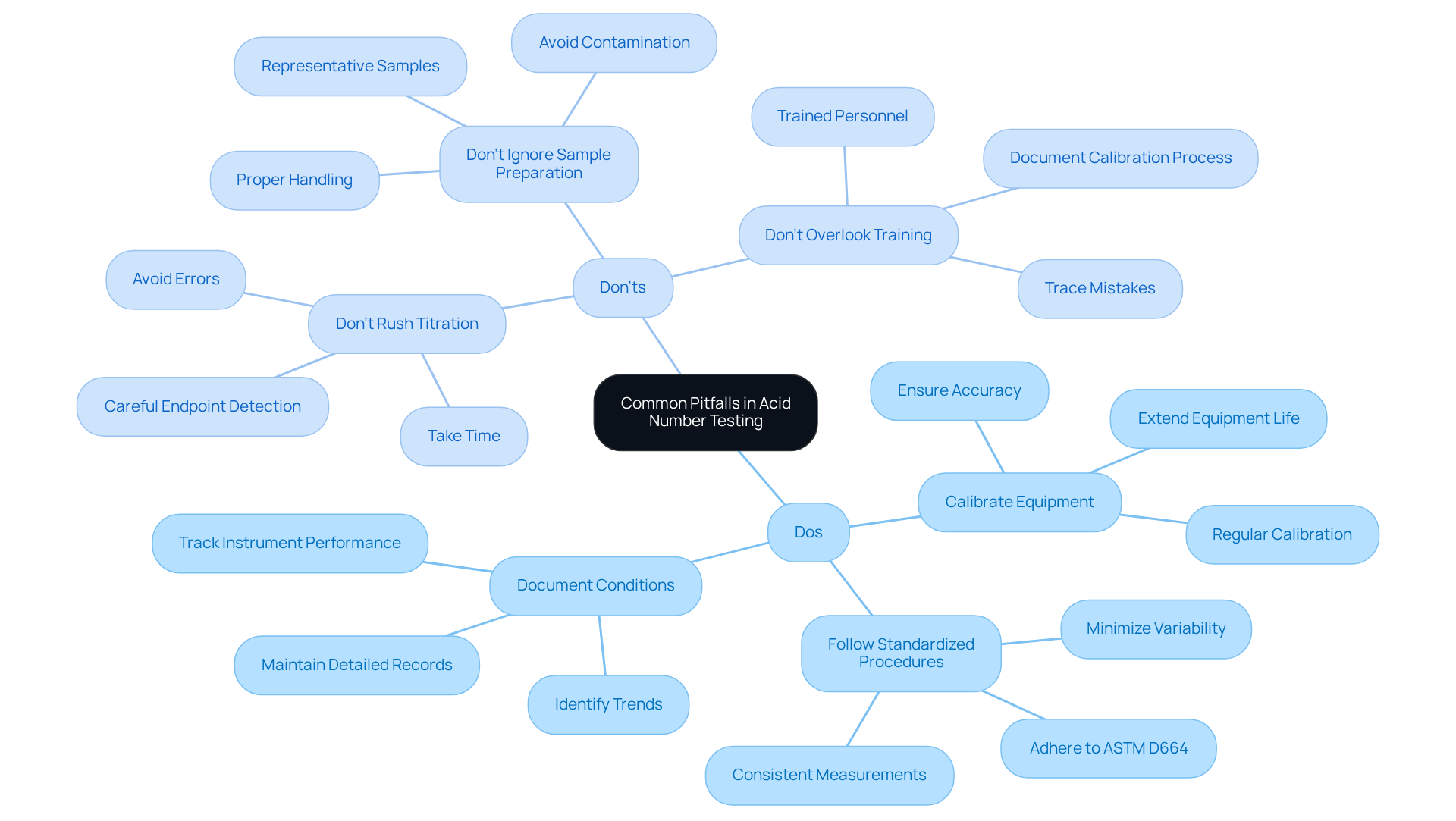
Common Pitfalls in Acid Number Testing: Dos and Don'ts
To guarantee precise acidity assessment, staff must remain vigilant against common mistakes.
Dos:
- Do calibrate equipment regularly to maintain accuracy; frequent calibration is essential, as uncalibrated instruments can lead to noisy data and unreliable results. Moreover, regular calibration can extend the useful life of expensive machinery, thus deferring capital expenditure on new equipment.
- Do follow standardized procedures to minimize variability; adherence to ASTM D664 ensures that measurements are consistent and reliable.
- Do document all examination conditions for future reference; maintaining detailed records aids in tracking instrument performance and identifying trends over time.
Don'ts:
- Don't rush the titration process, as this can lead to errors; a careful approach is necessary to achieve precise endpoint detection.
- Don't ignore sample preparation guidelines, as improper handling can compromise results; ensuring representative samples are taken is crucial for accurate analysis.
- Don't overlook the importance of training, as untrained personnel may introduce errors; individuals completing calibration need to be trained and are required to document the process to ensure any mistakes can be traced back to the source.
By adhering to these guidelines, laboratories can significantly enhance the reliability of their acidity assessments, ultimately contributing to improved maintenance strategies and operational excellence. Remember, a not only enhances product quality but also promotes safety and reduces downtime.
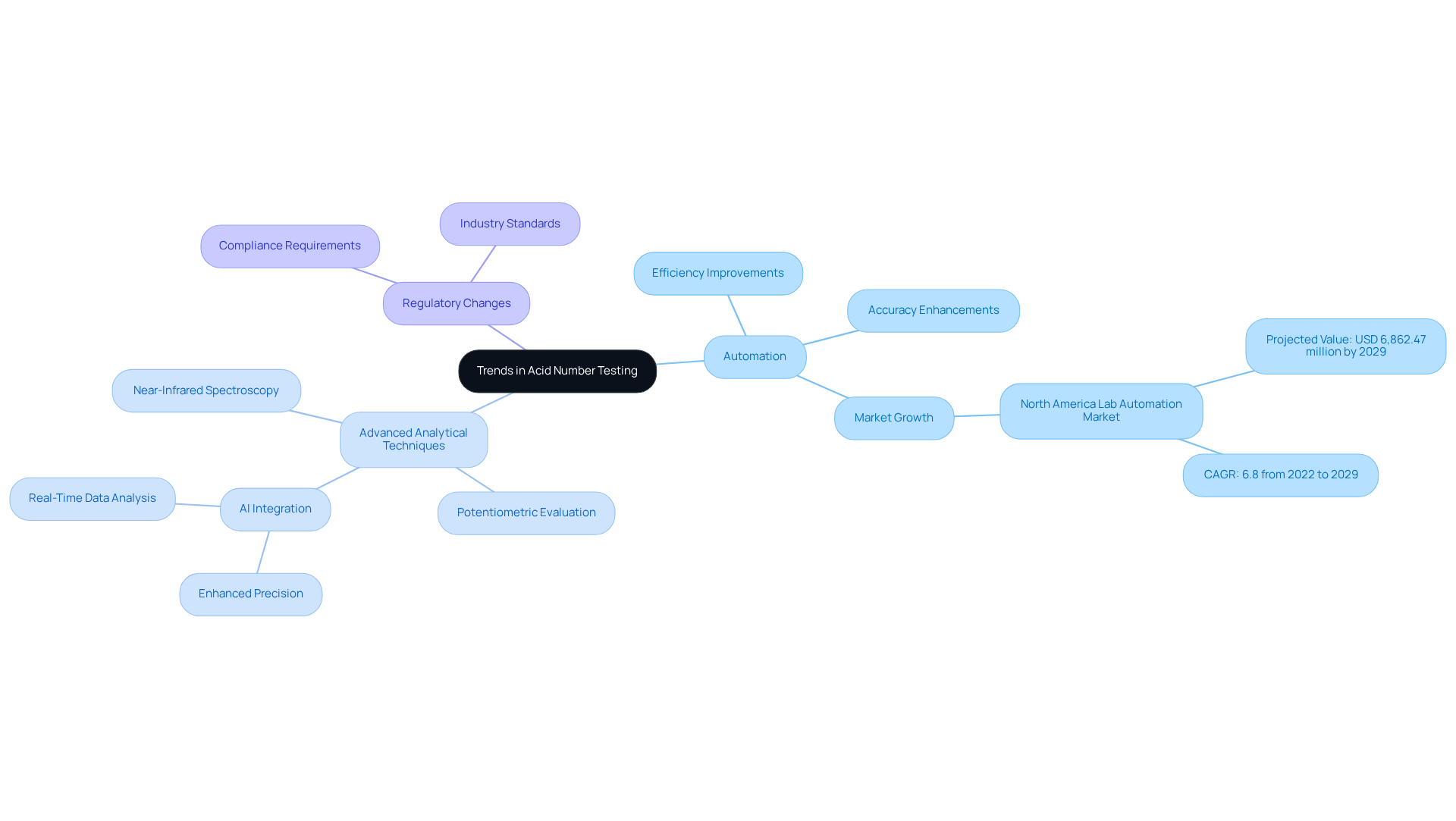
Trends in Acid Number Testing: What Laboratory Managers Should Know
Laboratory managers must recognize pivotal trends in acid number testing according to ASTM D664 that could profoundly influence their operations.
- Automation is at the forefront of this transformation. The integration of automated systems in testing processes is revolutionizing efficiency and accuracy. By streamlining workflows and minimizing human error, these systems lead to faster, more reliable results. As highlighted, "Automation has significantly reduced human error, enabling faster and more reliable results." This shift is crucial as facilities strive to enhance productivity and maintain high standards of quality control. The is anticipated to reach USD 6,862.47 million by 2029, expanding at a CAGR of 6.8% from 2022 to 2029, underscoring the importance of automation in research environments.
- Advanced Analytical Techniques are also gaining traction. Innovative methodologies, including near-infrared spectroscopy and potentiometric evaluation, are increasingly adopted for determining acidity number. These technologies provide rapid outcomes, allowing facilities to respond promptly to evaluation needs while ensuring measurement accuracy. Moreover, the incorporation of AI and automation technologies in the value analyzer market is enhancing both accuracy and efficiency in evaluation processes.
- Regulatory Changes present another critical aspect. As industry regulations evolve, it is essential for testing facilities to remain informed about compliance requirements related to acid number testing as specified by ASTM D664. Adapting to these changes not only ensures compliance with legal standards but also enhances the facility's credibility and operational integrity.
By staying informed about these trends, laboratory managers can strategically position their operations to be both competitive and compliant in an ever-evolving landscape.
Conclusion
The significance of ASTM D664 in pharmaceutical laboratories is paramount. This standard establishes a crucial framework for accurately determining the acid number of various products, thereby ensuring quality control and compliance with industry regulations. By implementing ASTM D664, lab managers can significantly enhance their testing processes, minimize variability, and uphold the integrity of their products.
Key insights throughout this article underscore the necessity of employing advanced titration technologies, adhering to standardized methodologies, and comprehending the implications of acid number limits. Additionally, the article emphasizes the critical need for proper calibration, staff training, and automation to bolster efficiency and accuracy in testing. Recognizing the distinctions between Total Acid Number and Acid Number is also vital for effective oil analysis and informed maintenance decision-making.
In conclusion, adopting ASTM D664 and its associated best practices strategically positions pharmaceutical laboratories for success in an increasingly competitive landscape. Lab managers are urged to remain vigilant about the latest trends, including advancements in automation and analytical techniques, to perpetually enhance their operations. By prioritizing rigorous testing standards and embracing innovation, laboratories can ensure product quality, maintain customer trust, and ultimately contribute to the advancement of the pharmaceutical industry.




