Overview
The article highlights the essential tools and step-by-step procedures for analyzing chromatograms, which are critical for ensuring the safety and efficacy of pharmaceutical products. Proper chromatogram analysis, supported by advanced technologies and systematic procedures, is crucial for accurately evaluating drug purity and compliance with regulatory standards. This meticulous approach not only safeguards public health but also reinforces the integrity of the pharmaceutical industry. As we delve into these practices, it becomes evident that the role of high-quality scientific instruments cannot be overstated. Their implementation is vital for maintaining the highest standards in laboratory settings.
Introduction
In the intricate realm of pharmaceutical analysis, chromatograms emerge as essential instruments that unveil the complexities inherent in chemical mixtures. These graphical representations not only delineate the separation of various components but also play a pivotal role in ensuring the safety and efficacy of pharmaceuticals. As the pharmaceutical industry advances, so too does the technology underpinning chromatographic techniques, empowering scientists to attain unprecedented precision in identifying active ingredients and impurities.
With innovations such as ultra-high-performance liquid chromatography and cutting-edge detection methods, the significance of chromatograms in upholding stringent regulatory standards has never been more pronounced. This article explores the critical importance of chromatograms, the essential tools required for effective analysis, step-by-step procedures for conducting tests, and troubleshooting common issues, ultimately underscoring their indispensable role in safeguarding public health.
Understand Chromatograms and Their Importance in Pharmaceutical Analysis
Analyzing chromatograms provides essential graphical representations that illustrate the separation of components within a mixture as they traverse a chromatographic system. In the field of drug analysis, these visual tools are indispensable for analyzing chromatograms, which help in recognizing and measuring active components, impurities, and degradation products. Each peak in a separation graph corresponds to a distinct component, which allows scientists to evaluate the purity and potency of drugs by analyzing chromatograms. This capability is crucial for adhering to regulatory standards and ensuring the safety and efficacy of pharmaceutical products.
The importance of analyzing chromatograms in evaluating drug safety and efficacy cannot be overstated. Recent advancements in chromatographic techniques, particularly the integration of high-resolution columns with multichannel detectors like mass spectrometers, have significantly enhanced molecular identification in complex mixtures. For instance, studies indicate that the likelihood of effective separation increases notably with the number of columns used—
- 25% with two columns
- 35% with three
- 44% with four
- 52% with five columns
This improvement is vital for addressing challenges such as overlapping peaks and complex sample matrices, which can obscure critical data.
In 2025, the significance of analyzing chromatograms in drug analysis continues to increase, with specialists highlighting their role in evaluating medication purity. The capability to precisely recognize and measure substances guarantees that medicinal products adhere to strict safety standards. Real-world applications illustrate this, as analyzing chromatograms is routinely employed to confirm the purity of active ingredients (APIs) and to observe the stability of drug formulations over time. The suitability test for drugs and medicines in compliance with the Japanese Pharmacopoeia is a critical aspect of this process, ensuring that all products meet necessary regulatory requirements.
Expert opinions emphasize that analyzing chromatograms is not just a tool but is crucial in protecting public health. As Akira Kotani notes, "the use of ultra-high-performance liquid chromatography (UHPLC) with UV detection exemplifies the advancements in repeatability assessment within HPLC systems, providing reliable data in a fraction of the time."
Overall, the existing trends in graphical representation examination demonstrate a dedication to innovation and accuracy in drug research, ensuring that medication safety and effectiveness stay at the forefront of scientific investigation. The incorporation of instruments such as the Hiranuma Aquacounter AQV-300 Volumetric and AQ-300 Coulometric Karl Fischer Titrators further improves the reliability of drug testing, aligning with chromatographic evaluation to ensure adherence to the Japanese Pharmacopoeia. This comprehension of separation diagrams not only enhances analytical skills but also assists laboratory managers in applying effective quality assurance practices.
Gather Essential Tools and Equipment for Chromatogram Analysis
To perform efficient chromatogram evaluation in drug laboratories, essential tools and equipment must be utilized effectively.
Chromatography System: This encompasses the chromatograph, whether High-Performance Liquid Chromatography (HPLC) or Gas Chromatography (GC), which is crucial for separating the components of your sample. HPLC is especially preferred in pharmaceutical examination due to its accuracy and adaptability.
Detectors: Essential detectors include UV-Vis, fluorescence, and mass spectrometry detectors. The introduction of Osaka Soda's NQAD, a revolutionary aerosol-based detector, significantly enhances this area. The NQAD utilizes proprietary technology to condense moisture on aerosol particles, enabling highly sensitive detection with superior accuracy compared to traditional detectors. This makes it invaluable for capturing overlooked components. Utilizing analytical software such as OpenChrom or Agilent OpenLab is critical for analyzing chromatograms and visualizing data. These tools facilitate the interpretation of complex data sets, allowing for more accurate results.
Preparation Tools: Proper preparation of materials is key to reliable results. This category includes vials, syringes, and filters, which are necessary for preparing samples before analysis.
Calibration Standards: Employing known standards for calibration is essential to ensure accurate measurements. This practice preserves the integrity of the evaluation and achieves reproducible results.
Safety Equipment: Personal protective equipment (PPE) such as gloves, goggles, and lab coats is essential to ensure safety during examination, safeguarding personnel from possible dangers linked to chemical handling.
AQ-300 Coulometric Karl Fischer Titrator: This instrument is vital for moisture determination in drug products, ensuring compliance with Japanese Pharmacopoeia standards.
The chromatography market is projected to grow significantly, particularly in the Asia Pacific region, driven by advancements in biotechnology and medicine. As the need for accurate analytical instruments grows, the significance of these vital tools in analyzing chromatograms cannot be emphasized enough. By leveraging the latest chromatography systems and technologies, including the innovative NQAD and AQ-300, pharmaceutical labs can enhance their analytical capabilities and ensure compliance with regulatory standards.
Follow Step-by-Step Procedures for Effective Chromatogram Analysis
To conduct effective chromatogram analysis, it is essential to follow these critical steps:
- Prepare Your Specimens: Ensure that specimens are meticulously prepared, filtered, and stored in appropriate vials to prevent contamination and degradation.
- Set Up the Chromatography System: Install the necessary columns and secure all connections. Calibrate the system with known standards to guarantee accuracy.
- Inject the Sample: Introduce your sample into the chromatograph using either an autosampler or manual injection, ensuring a consistent sample volume.
- Run the Analysis: Initiate the chromatographic run while vigilantly monitoring the system for any irregularities that may compromise results.
- Collect Data: Following the run, gather chromatographic data using analytical software, which will facilitate further examination.
- Analyze the Chromatogram: Identify peaks corresponding to different components and employ calibration curves for quantifying their concentrations. Ensure a stable baseline and well-defined peaks for reliable interpretation. A successful evaluation of the chromatographic output is indicated by a stable baseline, well-defined peaks, and adequate resolution between adjacent peaks.
- Document Results: Record findings meticulously, noting peak shapes, retention times, and any anomalies observed during the examination.
In pharmaceutical laboratories, the average time required for chromatogram evaluation can vary; however, efficient preparation and execution can significantly reduce this duration. For instance, a case study on robotic preparation of standards for chromatographic evaluation demonstrated that automation streamlines workflows, enabling scientists to focus on essential tasks while maintaining high accuracy standards. Adhering to best practices in specimen preparation and evaluation is crucial for obtaining trustworthy results and ensuring compliance with regulatory standards. As Dr. Sujatha Mahadevarao Premnath noted, "Healthcare team outcomes can be enhanced when using chromatography for medical diagnostics by ensuring effective interdisciplinary communication and proper training for all team members." Additionally, it is noteworthy that the first chromatography was performed by M. Tswett in 1906 using calcium carbonate as a stationary phase, marking a significant milestone in the evolution of this analytical technique.
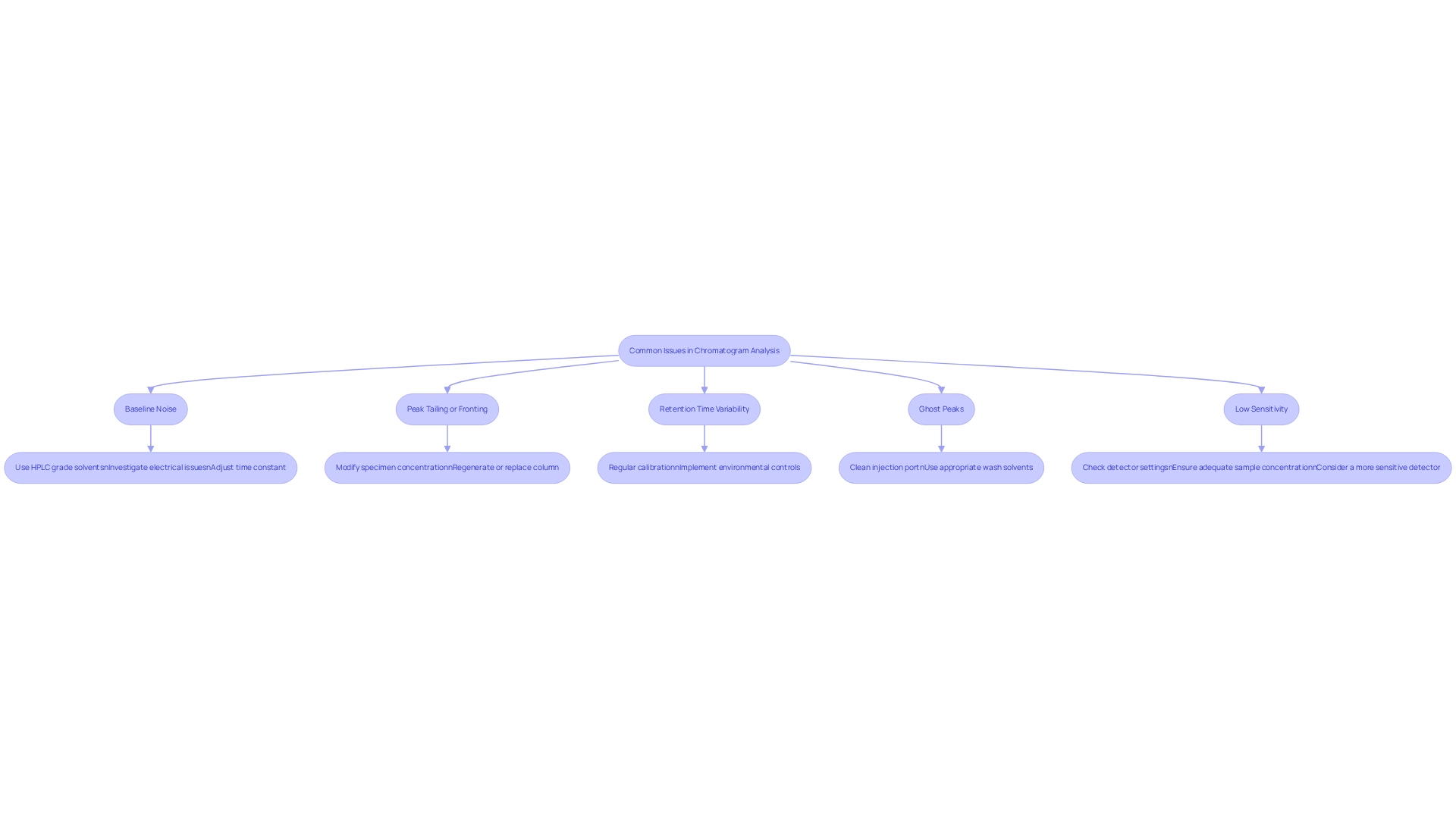
Troubleshoot Common Issues in Chromatogram Analysis
Common issues encountered while analyzing chromatograms can significantly impact the reliability of results. Addressing these prevalent challenges is crucial for ensuring accurate outcomes in laboratory settings.
- Baseline Noise often stems from electronic interference or subpar solvent quality, obscuring results. To mitigate this, ensure that solvents are of HPLC grade and investigate any potential electrical issues in the setup. Expert advice emphasizes the importance of adjusting the time constant to match peak width for optimal detector performance, thereby enhancing overall data clarity.
- Peak Tailing or Fronting typically signals column overload or issues with the stationary phase. Modifying the concentration of the specimen can assist in resolving these issues. However, if performance remains unsatisfactory after regeneration, substituting the column may be required. Real-world examples demonstrate that optimizing load management can significantly reduce peak distortion, leading to more reliable chromatographic results.
- Retention Time Variability can arise from temperature fluctuations or inconsistencies in the mobile phase. Regular calibration and maintenance of the system are essential to ensure stable conditions. Implementing environmental control measures, such as air conditioning, has proven effective in stabilizing laboratory conditions, as evidenced by case studies focused on HPLC stability.
- Ghost Peaks are unwanted signals often resulting from carryover from prior specimens. To prevent ghost peaks, thoroughly clean the injection port and utilize appropriate wash solvents between analyses. Implementing these practices can reduce ghost peak occurrences by up to 50%, enhancing the integrity of the data collected.
- Low Sensitivity presents a challenge when peaks are not detectable. It is vital to check the detector settings and confirm that the sample concentration is adequate. In some cases, employing a more sensitive detector may be necessary to enhance detection capabilities. By addressing these common issues with targeted solutions, laboratories can significantly enhance the accuracy and reliability of their analyses by analyzing chromatograms, ultimately leading to more precise results in their scientific endeavors.
Conclusion
The exploration of chromatograms within pharmaceutical analysis underscores their vital role in ensuring drug safety and efficacy. These graphical representations are indispensable for identifying and quantifying active ingredients, impurities, and degradation products, thus complying with stringent regulatory standards. Recent advancements in chromatographic techniques, including high-resolution columns and sophisticated detection methods, have markedly enhanced the capability to analyze complex mixtures, ensuring that pharmaceutical products consistently meet required safety standards.
Equipping laboratories with appropriate tools and adhering to systematic procedures are essential for effective chromatogram analysis. From chromatography systems and detectors to analytical software and sample preparation tools, each component is crucial in achieving reliable results. Furthermore, implementing best practices and addressing common issues can significantly boost the accuracy and efficiency of chromatographic tests, thereby supporting the overarching goal of safeguarding public health.
As the pharmaceutical industry continues to evolve, the significance of chromatograms will only increase. By harnessing innovative technologies and following rigorous analytical protocols, laboratories can uphold high standards of quality control. Ultimately, the commitment to precise chromatographic analysis not only strengthens the foundation of pharmaceutical safety but also reaffirms the industry’s dedication to public health and well-being.




