Overview
Chromatograms serve as essential visual instruments in scientific analysis, particularly within the pharmaceutical industry, where they play a pivotal role in identifying and quantifying the components of mixtures. This process is crucial for ensuring product quality and regulatory compliance. The article emphasizes the significance of chromatograms by illustrating their function in analyzing drug purity and stability.
Furthermore, it highlights recent advancements in chromatographic techniques that enhance operational efficiency and accuracy in laboratory environments. By understanding these developments, professionals can appreciate the necessity of high-quality scientific instruments, ultimately leading to improved outcomes in their analytical processes.
Introduction
In the realm of scientific analysis, chromatograms serve as essential tools that unlock the mysteries of complex mixtures. These graphical representations illuminate the separation of components and play a pivotal role in fields such as pharmaceuticals, where they ensure the quality and safety of products. As the analytical landscape evolves, the demand for precise and efficient chromatographic techniques continues to grow, driven by advancements in technology and an increasing emphasis on regulatory compliance. This article delves into the multifaceted world of chromatography, exploring its various methods and applications, as well as the challenges faced by laboratories today. Furthermore, it highlights future trends that promise to reshape the industry.
Defining Chromatograms: A Fundamental Overview
To understand chromatograms, one must recognize that they serve as a crucial visual representation of the separation of components within a mixture, generated through chromatographic techniques. This graphical output illustrates the detector response over time, with the x-axis denoting time and the y-axis reflecting the concentration of the separated components. Each peak within the chromatogram corresponds to a specific compound, enabling scientists to dissect and analyze the mixture's composition effectively. This raises the question of chromatograms and their significance across various scientific disciplines, particularly in pharmaceuticals, where they play a pivotal role in quality control and drug development.
The pharmaceutical analytical testing market is projected to reach $14.58 billion by 2030, growing at a compound annual growth rate (CAGR) of 8.41%. This growth underscores the increasing reliance on chromatographic methods for precise analysis and validation of pharmaceutical products. Laura Wood, Senior Press Manager, notes that the 'Membrane Chromatography Market Size, Share & Trends Analysis Report' has been added to ResearchAndMarkets.com's offerings, emphasizing the growing importance of separation techniques in the industry.
Real-world applications of chromatograms in pharmaceuticals are exemplified by case studies showcasing their role in ensuring product quality. For example, Doug McCabe, senior director at Waters Corporation, highlights the importance of separation techniques in quality control across various industries. He notes the challenges posed by complex separations and advocates for specialized techniques, alongside the trend of integrating cloud solutions for enhanced remote monitoring and data sharing.
This integration enhances flexibility and teamwork in laboratories while also enabling the adoption of advanced separation systems through user-friendly interfaces. Expert opinions further emphasize the significance of chromatograms in chemical analysis. Alan Owens, a seasoned product manager in gas analysis at , articulates that chromatograms are indispensable for interpreting analytical results, particularly in the context of pharmaceuticals.
They provide critical insights into the purity and composition of compounds, which are essential for regulatory compliance and product safety. JM Science Inc. supports pharmaceutical labs by offering a range of premium scientific instruments, including high-performance liquid chromatography (HPLC) systems, HPLC columns, Shodex Refractive Index and Conductivity detectors, and titrators. These advanced tools enhance the efficiency and accuracy of chromatographic analyses, ensuring that research facilities can effectively utilize chromatograms in their research and quality control processes. Additionally, JM Science offers competitive pricing on these products, making them accessible for various scientific needs.
In summary, chromatograms are vital tools that underpin scientific analysis and decision-making in the pharmaceutical industry. Their ability to convey complex information in an accessible format makes them invaluable for researchers and quality control professionals alike. To learn more about JM Science's offerings and how they can enhance your laboratory's capabilities, visit our website or contact us directly.
Exploring Different Types of Chromatography
This process represents a critical testing method encompassing several approaches, including gas separation (GC), liquid separation (LC), and high-performance liquid separation (HPLC). Each method operates on distinct principles designed to separate components based on their unique physical and chemical properties. Notably, GC excels in analyzing volatile compounds, making it a preferred choice in environmental and food safety testing.
Conversely, HPLC showcases versatility, accommodating a broader array of substances, including complex biomolecules, which is vital in pharmaceutical and biochemical research.
The choice of separation method significantly influences analytical outcomes, as each approach produces specific chromatograms that illustrate separation efficiency and resolution. Recent advancements in separation technology, particularly the emergence of fully automatic UHPLC machines equipped with sophisticated data management systems, have streamlined analysis processes, enhancing both accuracy and efficiency in laboratories.
As we look towards 2025, the market for separation methods is characterized by a notable shift towards innovative solutions, with a significant compound annual growth rate (CAGR) anticipated in the Academic & Research Institutes segment. This growth is propelled by ongoing technology launches, acquisitions, and robust research and development activities. Importantly, the market share of gas separation analysis remains competitive with liquid separation methods, with each approach serving unique applications across various scientific domains.
Expert opinions emphasize the indispensable role of HPLC in scientific inquiry, underscoring its importance in achieving accurate and reliable results. Anushka Gore, a Research Associate at Cognitive Market Research, observes, 'The progress in separation methods is essential for facilities striving to improve their analytical skills and fulfill the changing requirements of the scientific community.' Case studies comparing gas separation techniques and HPLC reveal that while GC excels in speed and efficiency for volatile substances, HPLC provides superior resolution for complex mixtures, making it crucial in pharmaceutical analysis and quality control.
JM Science Inc. plays a pivotal role in this landscape by offering a comprehensive range of , including high-performance HPLC columns, fittings, manual injection valves, solvent reservoir kits, and accessories that enhance research capabilities. Their commitment to quality and customer support, as highlighted in the case study titled 'Commitment to Quality and Customer Support,' showcases how the company enhances its value proposition through its product offerings and extensive support resources. Furthermore, JM Science provides competitive pricing on their products, making them an attractive option for facilities looking to optimize their budgets.
Statistics indicate that the use of various separation methods varies significantly among facilities, with HPLC favored for its versatility and accuracy. As research facilities increasingly aim to enhance their testing capabilities, understanding chromatograms is essential for selecting the most suitable technique between gas chromatography and liquid chromatography for specific research requirements. Additionally, JM Science's innovative medical devices, such as electronic stethoscopes, bolster modern medical practices by enabling remote patient monitoring, illustrating the company's diverse product range.
Applications of Chromatograms in Scientific Research
In the realm of scientific applications, especially within the pharmaceutical sector, chromatograms play a crucial role in analyzing drug purity and stability. High-Performance Liquid Chromatography (HPLC) chromatograms, bolstered by JM Science Inc.'s premium HPLC columns and accessories, are vital for identifying active pharmaceutical ingredients and detecting impurities, thus ensuring compliance with rigorous regulatory standards. Notably, recent statistics reveal that over 70% of pharmaceutical companies rely on chromatographic techniques for quality assurance, highlighting their significance in maintaining product integrity.
In addition to HPLC solutions, JM Science Inc. offers a wide array of scientific products, such as titrators and Karl Fischer reagents, which enhance testing capabilities in research facilities. The versatility of chromatograms transcends pharmaceuticals and environmental monitoring; they serve as indispensable tools in research, quality control, and regulatory compliance across various scientific disciplines, including nutraceuticals, food and beverages, and cosmetics.
Recent case studies underscore the challenges faced by facilities in the chemistry sector, including the high costs associated with advanced instruments and a skills gap in operating automated systems. These obstacles can impede smaller facilities from acquiring the essential tools needed to manage the increasing volume of data generated by analytical techniques. As Anushka Gore, a Research Associate at Cognitive Market Research, articulates, "The integration of innovative chromatographic methods is expected to revolutionize , ensuring that these critical applications remain at the forefront of scientific inquiry."
Nonetheless, ongoing advancements in chromatographic technology, including those provided by JM Science Inc., continue to bolster laboratory capabilities, enabling them to effectively meet the evolving demands of drug purity analysis and environmental monitoring.
As we advance further into 2025, expert opinions highlight the increasing importance of chromatograms in both pharmaceuticals and environmental science. The integration of innovative chromatographic methods, supported by JM Science's high-performance solutions, is anticipated to transform drug research and environmental assessments, ensuring that these critical applications remain pivotal in scientific inquiry. To discover more about JM Science's extensive range of products, including competitive pricing and innovative solutions, visit our website today!
Interpreting Chromatograms: Key Metrics and Analysis Techniques
A fundamental aspect of lies in interpreting chromatograms, which necessitates a thorough analysis of key metrics such as retention time, peak area, and peak height. Retention time is crucial; it indicates the duration a compound takes to traverse the chromatographic system, serving as a fingerprint for identifying substances. Conversely, peak area is directly proportional to the quantity of the analyte present, making it essential for quantification.
Resolution, defined as the ability to differentiate between two closely eluting peaks, plays a pivotal role in ensuring accurate analysis. High resolution is vital for distinguishing compounds that may exhibit similar retention times, thereby enhancing the reliability of results. Recent advancements in separation methods have introduced innovative approaches such as baseline correction and peak integration, significantly enhancing data interpretation. These methods empower scientists to refine their analyses, ensuring precise quantification and identification of compounds.
In 2025, the importance of these metrics is underscored by the projected growth in the Academic & Research Institutes segment, which is expected to expand at a significant compound annual growth rate (CAGR). This growth emphasizes the rising dependence on separation methods for diverse applications, including pharmaceuticals and environmental testing.
Real-world instances of peak integration processes illustrate their practical use in analysis. For instance, in a study investigating the separation of complex mixtures, researchers successfully employed advanced peak integration methods to enhance the precision of their results. This showcases the effectiveness of these techniques in optimizing evaluation processes and aligns with findings from the case study titled 'Understanding Columns,' which emphasizes the critical role of these columns in achieving effective separation.
Expert opinions further reinforce the significance of retention time and peak area in comprehending chromatograms. Anushka Gore, a Research Associate at Cognitive Market Research, observes that a comprehensive understanding of these metrics is essential for optimizing separation processes and achieving dependable results. As the field of separation science continues to evolve, staying abreast of recent techniques and methodologies will be crucial for laboratory managers aiming to enhance their testing capabilities.
Moreover, the competitive landscape features strategic initiatives, such as new product launches, which are vital for advancing separation technologies and meeting the demands of the scientific community.
Technological Innovations in Chromatography
Recent advancements in separation technology have significantly transformed the landscape of analytical science. One of the most notable innovations is the emergence of automated systems, which streamline sample preparation and analysis processes. These systems not only minimize human error but also enhance throughput, allowing laboratories to handle larger volumes of samples with greater efficiency.
The integration of automation has been shown to enhance operational efficiency by reducing processing times and increasing the consistency of results. Moreover, the coupling of mass spectrometry with other analytical techniques has revolutionized detection capabilities. This combination enhances both sensitivity and specificity, enabling the identification of trace levels of compounds that were previously challenging to detect.
As a result, the applications of this separation technique have expanded significantly, making it an essential tool across various fields, including pharmaceuticals, life sciences, food and beverages, and environmental analysis.
The impact of these technological innovations is underscored by industry experts who emphasize the importance of adapting to these advancements. For example, Alan Owens, GC/GC-MS Product Manager, observes that decreased power usage and operational expenses have become key to product development in this field. Furthermore, the movement towards , as emphasized by industry leaders, enables simpler adoption of advanced methods across laboratories of all expertise levels.
This shift not only improves operational efficiency but also enhances the quality control processes in industries dealing with complex separations, such as biopharmaceuticals and environmental testing. Statistics indicate that the global market for separation techniques is poised for substantial growth, with a projected compound annual growth rate (CAGR) of 30.9% from 2025 to 2032. This growth illustrates the rising need for advanced methods and the ongoing development of separation technology.
Moreover, Doug McCabe, senior director at Waters Corporation, highlights the significance of this process in quality control across multiple sectors, especially in tackling the difficulties presented by intricate separations. The trend towards standardized, preconfigured chromatography systems and cloud integration enhances accessibility and reduces human error, allowing labs of all expertise levels to adopt advanced techniques and improve operational efficiency. In summary, understanding chromatograms is essential, as the latest innovations in chromatography, particularly in automation and mass spectrometry, are reshaping the field, driving efficiency, and expanding the range of applications. These advancements are vital for research facilities seeking to improve their evaluation abilities and satisfy the increasing requirements of scientific inquiry.
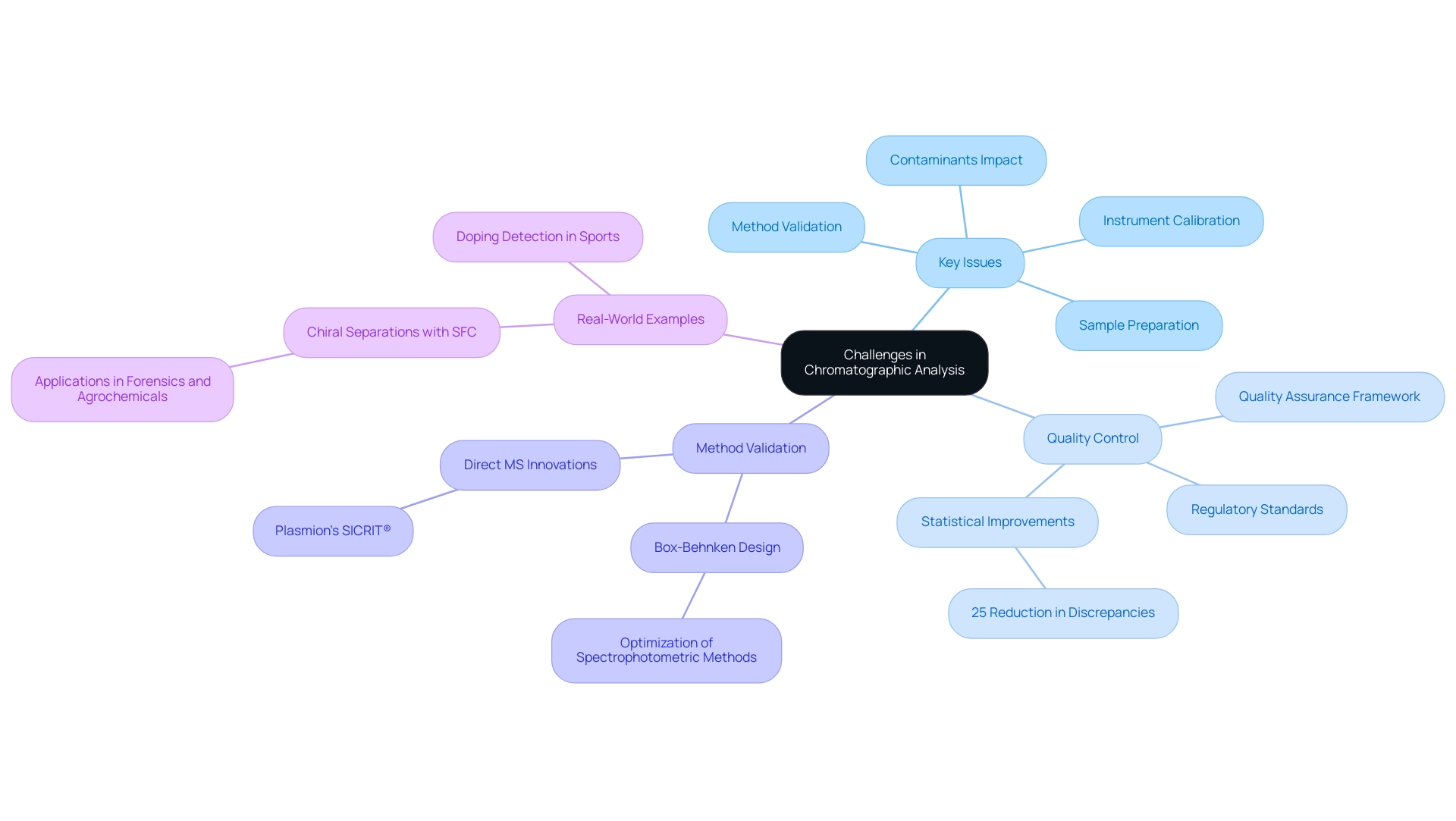
Challenges in Chromatographic Analysis and Quality Control
The challenges in chromatographic analysis prompt a critical examination of chromatograms and their substantial influence on the accuracy and reliability of results. Key issues encompass sample preparation, method validation, and instrument calibration. The integrity of samples is paramount; even minor contaminants can distort results, leading to erroneous conclusions.
A recent study revealed that nearly 30% of chromatographic errors arise from inadequate sample handling, underscoring the necessity for meticulous preparation protocols. Moreover, adherence to regulatory standards demands stringent quality control measures. Regular calibration of instruments and thorough validation of methods are essential components of a robust quality assurance framework. For instance, the application of Box-Behnken design in optimizing spectrophotometric methods has shown significant improvements in quantifying banned substances, illustrating the role of chromatograms and how method validation can enhance measurement precision.
This is particularly relevant in the context of quantifying acetazolamide, a drug banned by WADA, where optimized methods contribute to improved monitoring techniques. In the realm of quality control, its importance cannot be overstated. Statistics indicate that laboratories employing rigorous quality control protocols experience a 25% reduction in analytical discrepancies. This is especially critical in industries such as pharmaceuticals, where the implications of inaccurate data can be profound.
Recent advancements in separation techniques and mass spectrometry have further strengthened the reliability of drug testing, particularly in sports, where understanding chromatograms is essential for doping detection. Notably, separation techniques and mass spectrometry are being utilized to detect doping in athletes, emphasizing their reliability in .
Real-world examples illustrate these challenges and solutions. A notable case study on chiral separations using supercritical fluid chromatography (SFC) has expanded its applications beyond pharmaceuticals into forensics and agrochemicals, reflecting the versatility and necessity of method validation in diverse analytical contexts. Additionally, advancements in Direct MS with Plasmion’s SICRIT® highlight ongoing innovations in the field, as noted by Jan Wolf.
As the field evolves, research facilities must remain vigilant, continuously updating their practices to meet the dynamic demands of scientific inquiry and regulatory compliance. By implementing comprehensive quality assurance protocols, laboratories can ensure the reliability and reproducibility of chromatographic analyses, ultimately contributing to advancements in research and healthcare.
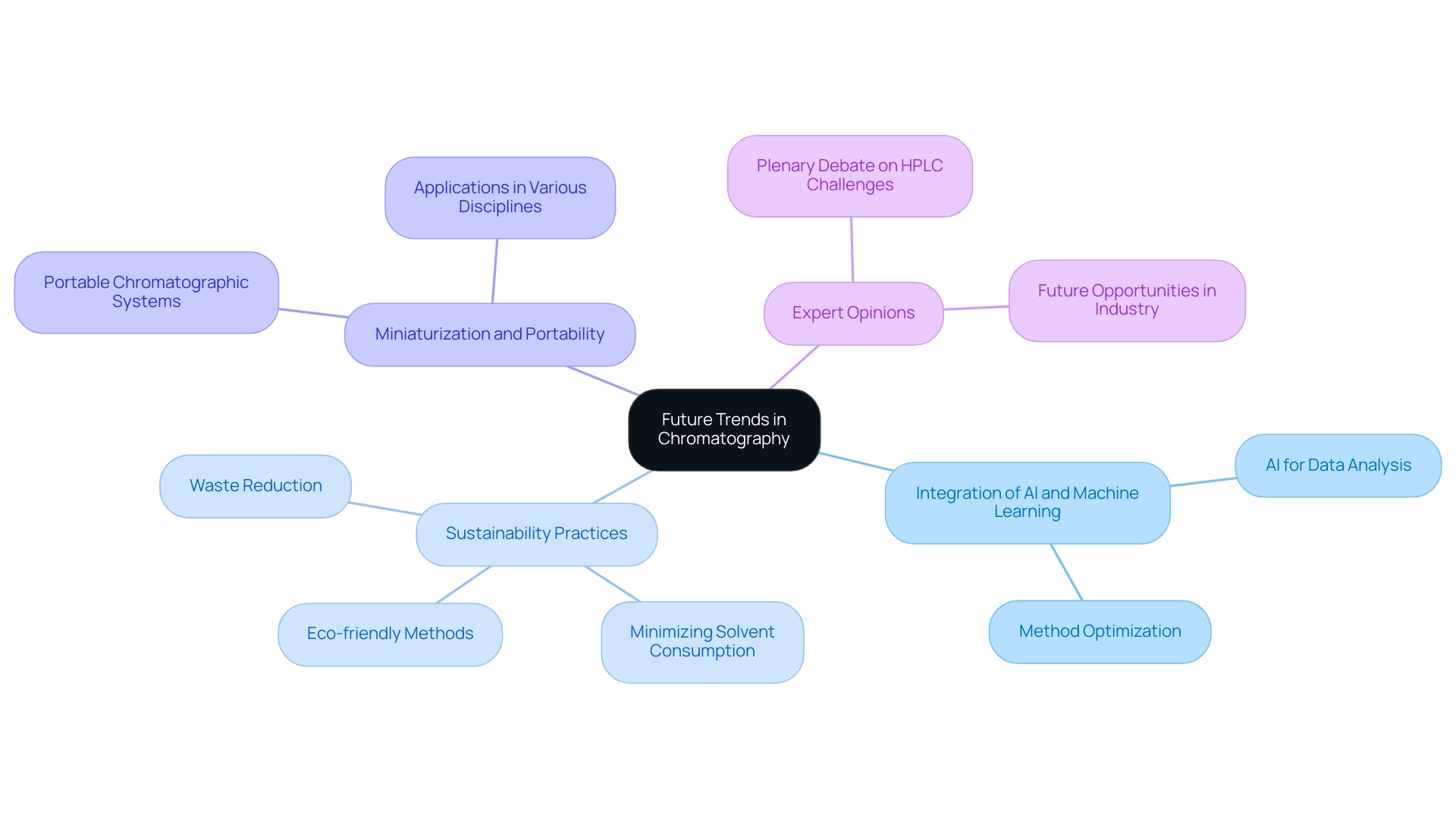
Future Trends in Chromatography: What Lies Ahead
The future of chromatography is poised for remarkable transformations, driven primarily by the integration of artificial intelligence (AI) and machine learning in data analysis and method optimization. These technologies streamline processes while enhancing the accuracy and efficiency of chromatographic methods. For instance, AI-driven algorithms are increasingly utilized to analyze complex chromatographic data, facilitating faster decision-making and improved method development.
Sustainability emerges as another critical focus area, with innovative approaches aimed at minimizing solvent consumption and reducing waste generation. The sector is witnessing a rise in eco-friendly methods, essential for complying with environmental regulations while preserving evaluation performance. A topical poster competition assessing methods with green metrics exemplifies this trend, showcasing the commitment to sustainable practices within the field.
Moreover, the demand for rapid and efficient assessment techniques propels the development of miniaturized and portable chromatographic systems. These advancements not only enhance the capabilities of chromatographic methods but also expand their applications across diverse scientific disciplines, from pharmaceuticals to environmental monitoring.
Expert opinions underscore that the future of separation techniques will be characterized by a seamless blend of technology and sustainability. As analytical chemistry evolves, the integration of AI and green methodologies will play a pivotal role in addressing the complex challenges laboratories face today. According to an unnamed speaker, "Another cornerstone in the industry-oriented part of the program will be a plenary debate on the current challenges and future opportunities for HPLC analysis in industry."
This reflects the ongoing dialogue within the community regarding the direction of separation techniques.
Despite existing hurdles such as high costs and skills gaps, the continuous development of innovative solutions ensures that remains vital in meeting the demands of modern society. The program's emphasis on sustainability, lab automation, data processing, and preparative chromatography further highlights the industry's commitment to evolving practices that align with contemporary needs.
Conclusion
In the dynamic landscape of scientific analysis, chromatograms are indispensable tools that facilitate the understanding of complex mixtures across various fields, particularly in pharmaceuticals. This article has explored the fundamental aspects of chromatograms, detailing their significance in ensuring product quality, safety, and regulatory compliance. With the pharmaceutical analytical testing market projected to see substantial growth, the reliance on chromatographic techniques is more critical than ever.
The discussion on different chromatography methods, including gas chromatography and high-performance liquid chromatography, highlights how each technique serves unique applications and contributes to improved analytical outcomes. The integration of advanced technologies, such as automation and mass spectrometry, has revolutionized laboratory capabilities, enhancing efficiency and accuracy in data interpretation. However, alongside these advancements, challenges persist in sample preparation, method validation, and adherence to strict quality control measures.
Looking ahead, the future of chromatography promises exciting developments driven by innovations in AI and sustainability. These trends indicate a shift towards more efficient and environmentally friendly practices, ensuring that laboratories can adapt to the evolving demands of scientific inquiry. As the industry continues to embrace these changes, the importance of chromatograms will only grow, underscoring their role as foundational elements in analytical chemistry.
Ultimately, the multifaceted nature of chromatography not only highlights its current relevance but also positions it as a cornerstone for future advancements in various scientific disciplines. As laboratories strive to enhance their capabilities and meet rigorous standards, chromatograms will remain at the forefront of analytical methodologies, paving the way for breakthroughs in research and quality assurance.




