Overview
The article delineates four essential practices for effectively packing columns in pharmaceutical labs. These practices encompass the following:
- Establishment of effective packing techniques
- Implementation of routine maintenance protocols
- Assurance of compliance with regulatory standards
- Troubleshooting of common packing issues
Each practice is underpinned by detailed procedures and recommendations. For instance, selecting appropriate stationary phases is crucial, as is maintaining meticulous records for compliance. Additionally, addressing specific challenges, such as channeling and flow inconsistencies, is vital for enhancing the overall performance and reliability of chromatography systems.
Introduction
In the intricate world of chromatography, the success of analytical processes hinges on mastering a few key techniques. Establishing effective column packing methods and troubleshooting common issues are essential steps that play a crucial role in achieving reliable results.
Laboratories must navigate a landscape filled with regulatory standards and maintenance protocols, all while ensuring the highest quality of data.
This article delves into essential practices that empower laboratory professionals to optimize their chromatography systems, enhance performance, and maintain compliance. By exploring these strategies, readers will gain valuable insights into the art and science of chromatography, paving the way for more efficient and effective analytical endeavors.
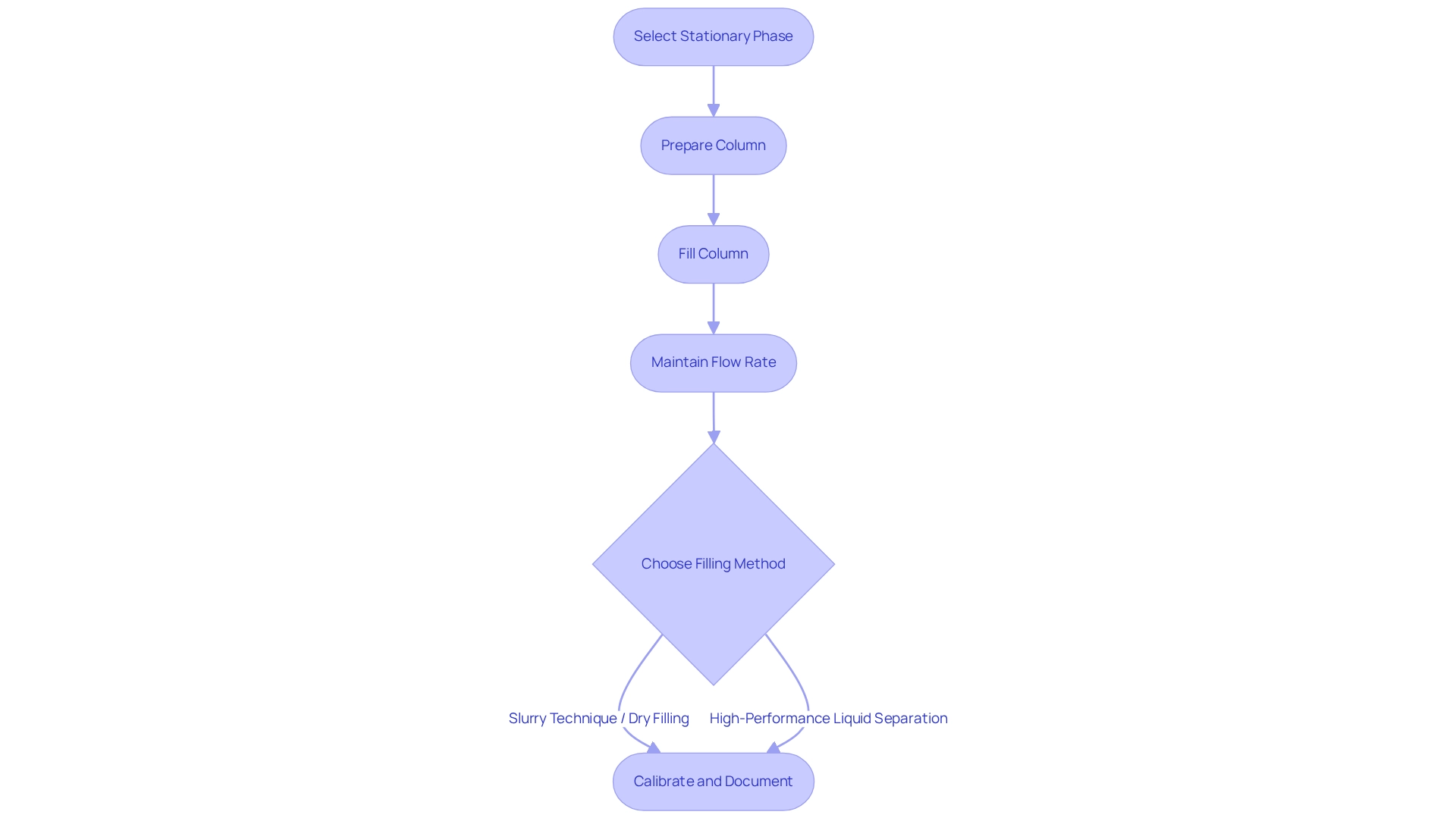
Establish Effective Column Packing Techniques
To create efficient support material techniques, begin by selecting the appropriate stationary phase substance, such as silica or alumina, tailored to the specific application. Preparing the column is crucial; ensure it is clean and dry before filling it with a slurry of the stationary phase combined with a suitable solvent. Maintaining a consistent flow rate throughout the process is essential to prevent channeling and to guarantee a uniform bed height.
Depending on the type of separation process being executed, methods like the slurry technique or dry filling can be employed. For example, in high-performance liquid separation, filling the tube under pressure is a common practice to achieve a dense and stable arrangement.
Regular calibration of the packing process, along with meticulous documentation of parameters such as flow rates and bed heights, can significantly enhance reproducibility and overall performance.
Implement Routine Maintenance Protocols
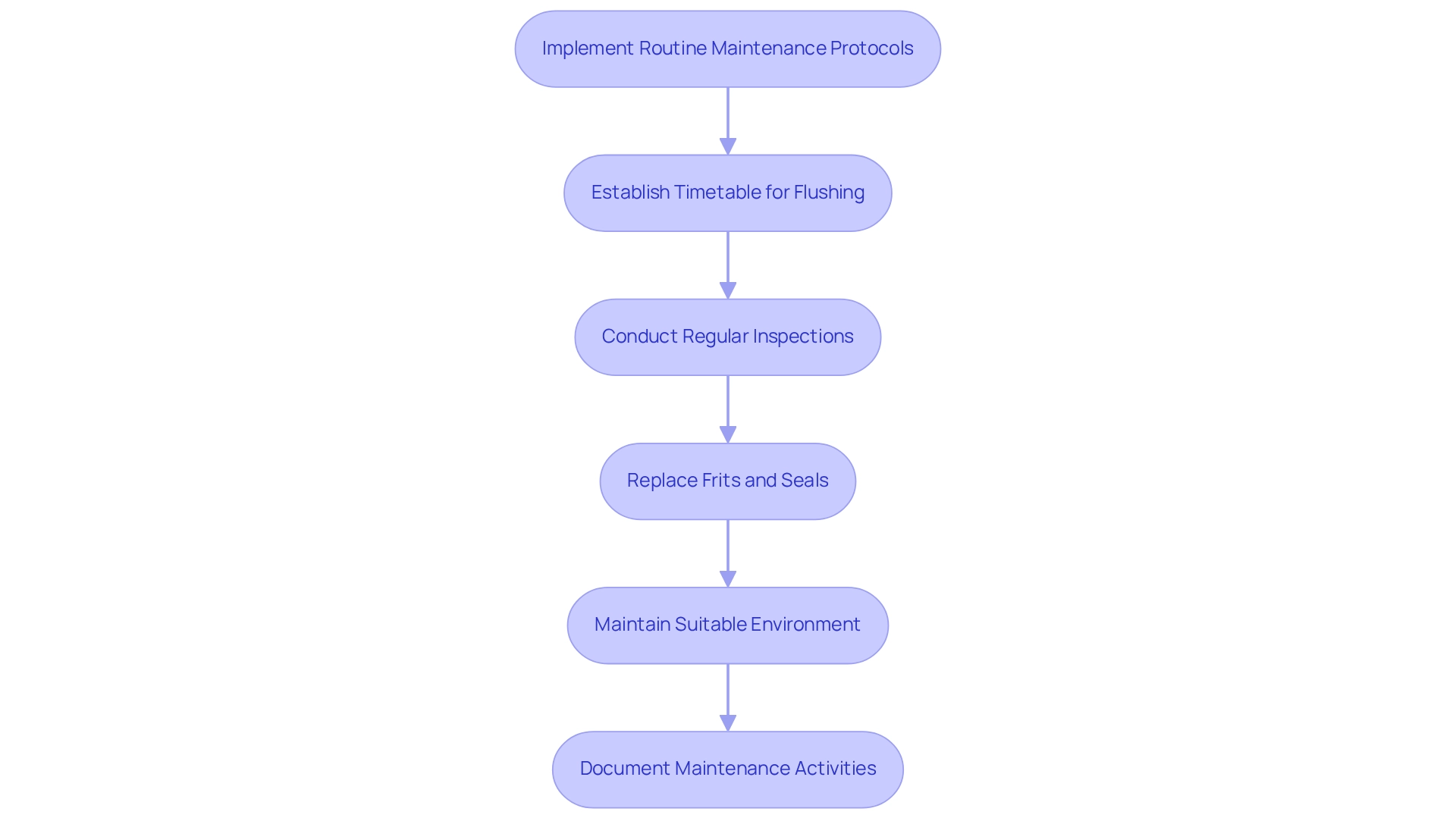
Implementing routine maintenance protocols is essential for ensuring the longevity and performance of chromatography equipment in pharmaceutical laboratories. Establishing a consistent timetable for flushing structures with appropriate solvents is vital to eliminate residual materials after each use. This practice not only prevents contamination but also enhances the precision of analytical results, as evidenced by the case study on supercritical fluid systems. This case underscores the necessity of flushing the system and ensuring proper connections to avert operational issues.
Regular inspections for signs of wear, including cracks or blockages, are imperative. Timely replacement of frits and seals can avert leaks and sustain optimal flow rates, which are crucial for generating reliable data. Additionally, maintaining the sections in a suitable environment—preferably within a solvent that prevents drying—can significantly extend their lifespan.
Documenting maintenance activities and outcomes represents another best practice. This documentation enables lab technicians to monitor performance metrics and discern patterns that may signal underlying issues, thereby facilitating timely interventions. As Joe Har remarked, "Your proactive approach to data analysis and statistical process control will enable smarter maintenance decisions, ultimately improving laboratory efficiency and research quality." Furthermore, leveraging digital documentation systems can simplify data retrieval and trend analysis, reinforcing the necessity of maintaining comprehensive records of maintenance activities. By adhering to these protocols, pharmaceutical labs can ensure that their packing columns operate at peak performance, contributing to the overall success of their analytical endeavors.
Ensure Compliance with Regulatory Standards
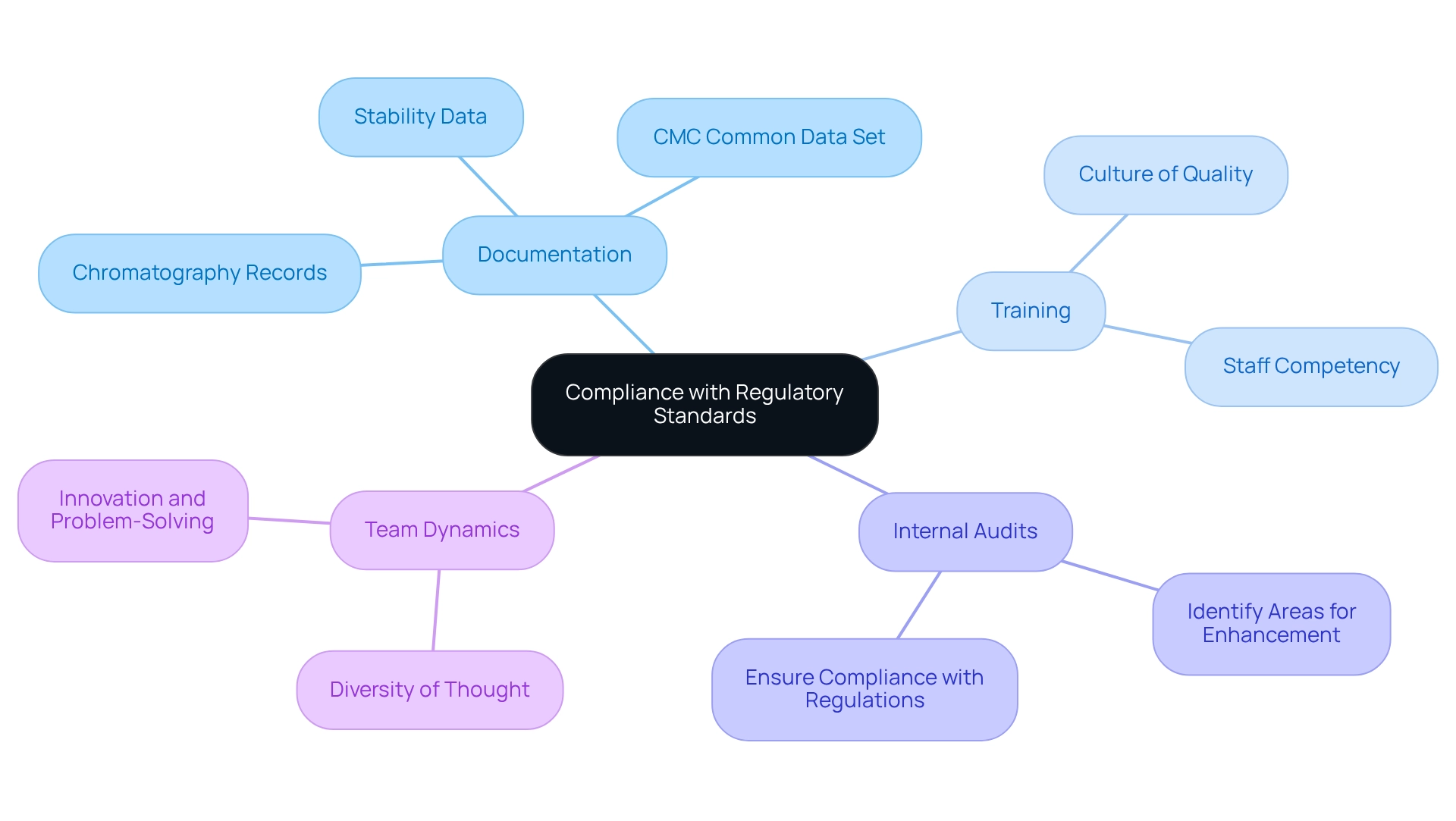
To achieve compliance with regulatory standards, laboratories must rigorously follow guidelines established by the FDA and EMA. This involves maintaining comprehensive records of all chromatography processes, including detailed procedures for the packing column, maintenance logs, and performance metrics.
The CMC Common Data Set, which includes information about the drug’s chemical composition, manufacturing processes, and stability data, underscores the importance of meticulous documentation in confirming product stability under normal storage conditions.
Regular training for laboratory personnel is crucial to instill a culture of quality and accountability, ensuring that staff are well-versed in compliance requirements. As Vivianne Arencibia, Vice President of Global Quality Systems and Compliance, states, "You need people. They need to be your nucleus."
Moreover, performing internal audits is a useful approach to identify areas for enhancement and ensure that procedures conform to current regulations. The use of validated methods and equipment that meet industry standards is essential for sustaining compliance and enhancing the reliability of laboratory results.
Regulatory agencies increasingly require detailed stability data to confirm that products remain stable under normal storage conditions, underscoring the importance of meticulous documentation and adherence to established protocols.
Furthermore, encouraging a variety of viewpoints within laboratory teams can stimulate innovation and enhance compliance methods, as diverse perspectives lead to more efficient problem-solving. The insights shared in the case study "Personal Path to Success" highlight the importance of resilience and curiosity in maintaining high standards in laboratory practices.
Troubleshoot Common Column Packing Issues
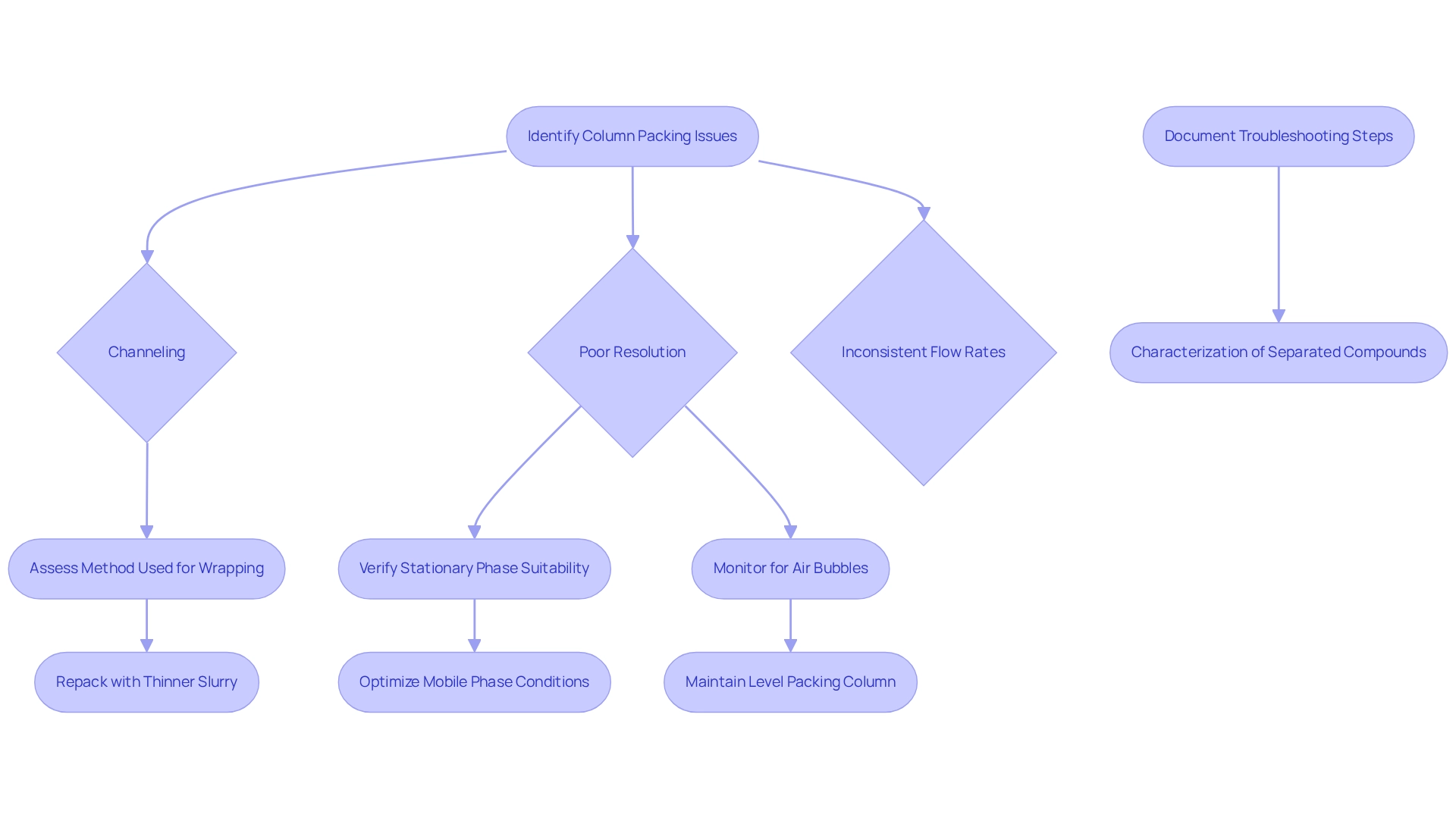
Common column arrangement issues in chromatography, including channeling, poor resolution, and inconsistent flow rates, can significantly impact analytical outcomes. To effectively troubleshoot these problems, begin by assessing the method used for wrapping. Improper slurry preparation or insufficient compression pressure often results in uneven distribution, leading to channeling. If channeling is observed, repacking the apparatus with a thinner slurry can promote uniformity.
For resolution challenges, verifying that the stationary phase is suitable for the analytes being separated and ensuring that the mobile phase conditions are optimized for the specific application is crucial. Regular monitoring for air bubbles during the packing process and maintaining a level packing column can prevent many common issues from arising.
As Dr. Jessica Torres, Project Manager in Ultrafiltration, aptly states, "There’s nothing like having the practice of doing it yourself to know how to tackle issues and troubleshoot a procedure." This underscores the significance of practical experience in addressing separation technique challenges.
Documenting each troubleshooting step and its outcomes not only aids in refining the packing column but also serves as a valuable reference for future analyses. A pertinent example is the characterization of separated compounds post-fluid catalytic cracking (FCC), illustrating the importance of employing various analytical methods to gain insights into the molecular nature of compounds. This ultimately informs decisions in both research and industrial applications. By following these optimal methods, laboratory supervisors can enhance the dependability and effectiveness of their separation systems. Moreover, for further insights, the eBook with top tips from the researcher community is available for download, providing valuable resources for best practices in chromatography.
Conclusion
Establishing effective column packing techniques, implementing routine maintenance protocols, ensuring compliance with regulatory standards, and troubleshooting common issues are pivotal to the success of chromatography in laboratory settings. By selecting the right stationary phase and employing consistent packing methods, laboratories can significantly enhance the reliability of their analytical results. Regular maintenance not only prolongs the lifespan of chromatography columns but also ensures optimal performance, thereby reducing the risk of contamination and inaccuracies.
Moreover, adherence to regulatory standards through meticulous documentation and training fosters a culture of quality and accountability within laboratory teams. This commitment to compliance not only meets the expectations of governing bodies like the FDA and EMA but also enhances the overall reliability of laboratory findings.
Finally, the ability to troubleshoot common packing issues effectively can save time and resources while improving analytical outcomes. Documenting troubleshooting efforts and outcomes serves as a valuable reference, promoting continuous improvement in laboratory practices.
In summary, mastery of these essential chromatography practices empowers laboratory professionals to optimize their systems, enhance performance, and maintain the highest quality of data. By embracing these strategies, laboratories can pave the way for more efficient and effective analytical endeavors, ultimately contributing to the advancement of scientific research and quality assurance in the pharmaceutical industry.




