Overview
This article provides essential insights into the significance and implications of capacity factor in high-performance liquid chromatography (HPLC), specifically tailored for lab managers. Understanding and optimizing the capacity factor is not just beneficial; it is critical for enhancing separation efficiency and achieving dependable analytical results. Various practical applications and case studies illustrate how careful adjustments and innovations have led to substantial improvements in chromatographic techniques. By focusing on these aspects, lab managers can drive advancements in their operations and ensure the reliability of their analytical outcomes.
Introduction
High-performance liquid chromatography (HPLC) is at the forefront of analytical chemistry, where precision and efficiency are paramount. The capacity factor serves as a critical performance metric, playing a vital role in determining the effectiveness of separations. This metric impacts a wide range of applications, from pharmaceutical development to environmental testing.
As laboratories strive for higher accuracy and reproducibility, understanding the intricacies of the capacity factor becomes essential. Lab managers face significant challenges in optimizing this key parameter. They must navigate the complexities of HPLC technology while leveraging the latest advancements to enhance their analytical outcomes.
How can they effectively address these challenges?
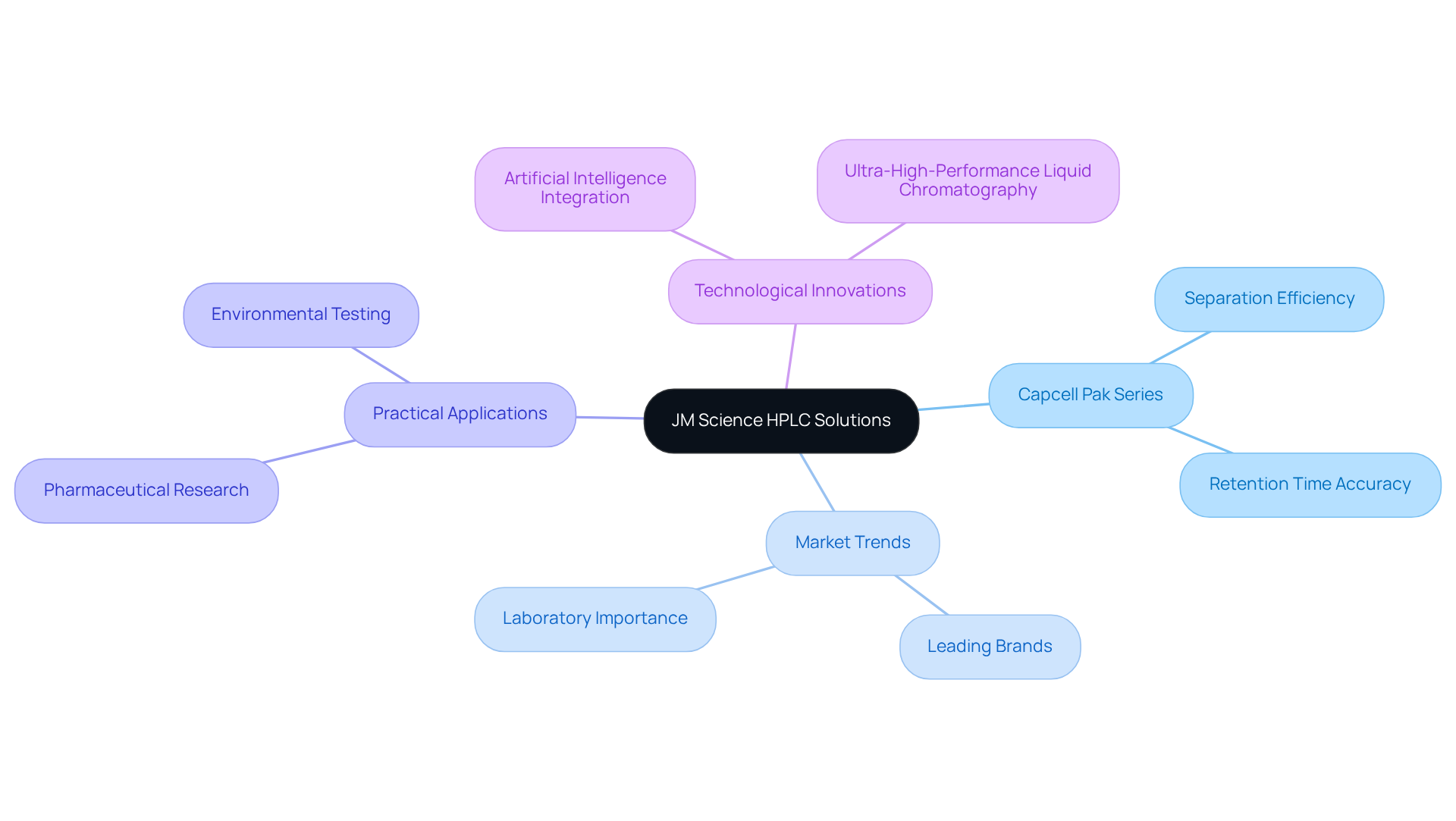
JM Science HPLC Solutions: Advanced Instruments for Capacity Factor Analysis
JM Science Inc. offers a comprehensive range of high-performance liquid chromatography instruments specifically crafted for precise capacity factor HPLC analysis. Notably, the Capcell Pak series distinguishes itself with its outstanding design, engineered to maximize separation efficiency and improve retention time accuracy. These sophisticated are indispensable for laboratories aiming to elevate the reproducibility of their analytical results, thereby supporting rigorous quality control and compliance with regulatory standards.
The market for high-performance liquid chromatography columns remains robust, with leading brands commanding significant shares, underscoring their vital importance in laboratory settings. The Capcell Pak series, in particular, is gaining recognition for its reliability and performance, making it a favored option among laboratory managers. Recent innovations in high-performance liquid chromatography technology, particularly the incorporation of artificial intelligence, have further augmented capacity factor HPLC analysis capabilities, facilitating quicker method development and enhancing analytical precision.
Practical applications of these advanced chromatographic columns illustrate their effectiveness across diverse laboratory environments. For instance, Capcell Pak columns are utilized in pharmaceutical research, resulting in more accurate drug formulation analyses, while environmental laboratories employ them for sensitive contaminant testing. Industry leaders emphasize the importance of such advancements, noting that progress in high-performance liquid chromatography technology is essential for meeting the evolving demands of analytical chemistry.
As the high-performance liquid chromatography market continues to grow, driven by increasing investments in research and development, JM Science remains committed to providing high-quality solutions that empower laboratories to achieve their analytical goals efficiently.
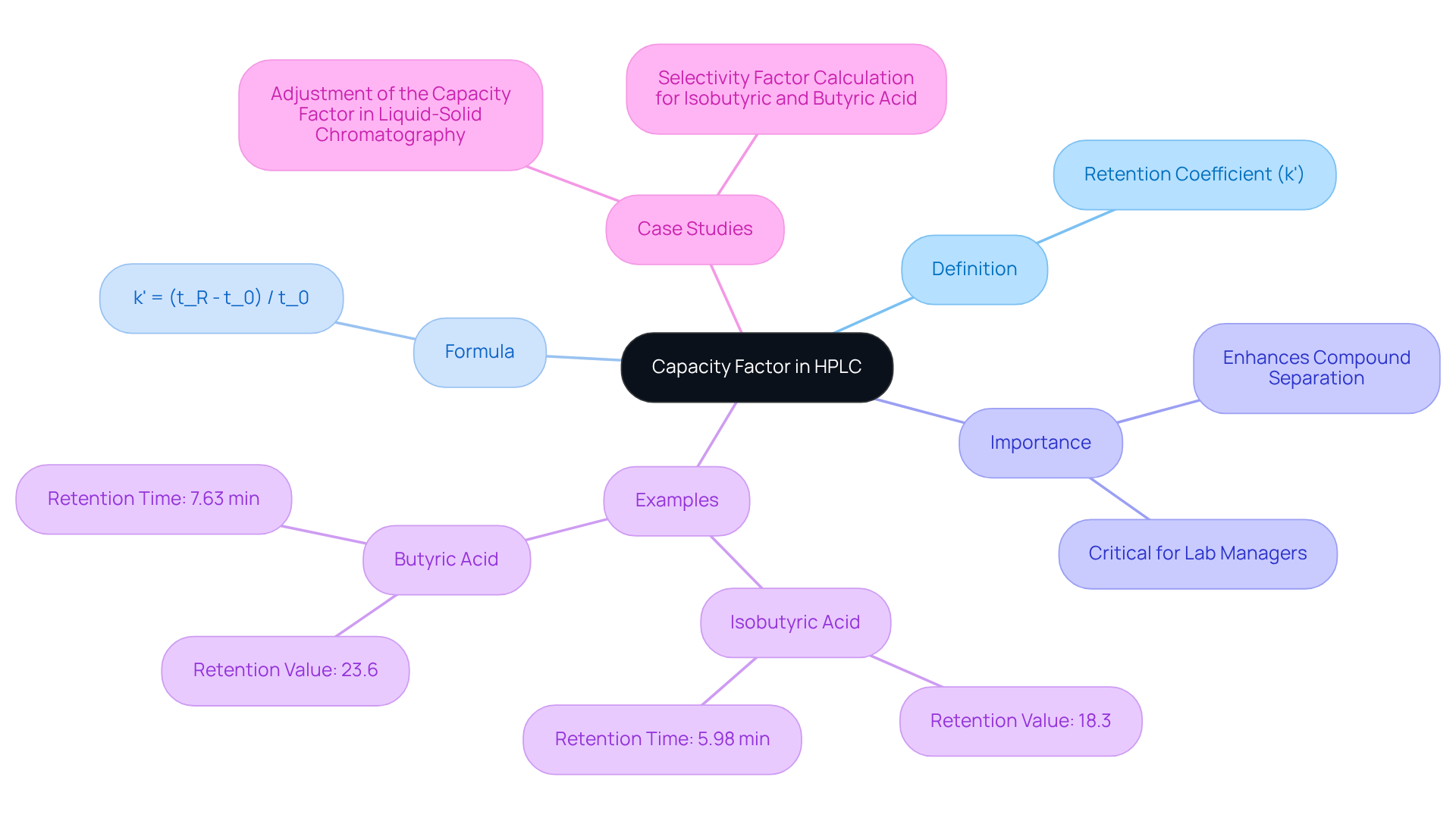
Understanding Capacity Factor: Definition and Importance in HPLC
The performance metric, widely recognized as the retention coefficient (k'), serves as a pivotal measurement in high-performance liquid chromatography (HPLC). It evaluates the retention of an analyte on the column in relation to the mobile phase. This metric is calculated as the to the time it spends in the mobile phase. Specifically, the performance ratio is expressed as
k' = (t_R - t_0) / t_0
where t_R denotes the retention time of the analyte and t_0 represents the void time of the column. A higher performance measure signifies enhanced retention, critical for effective compound separation. This understanding empowers lab managers to refine their methodologies, thereby improving resolution and accuracy in analytical results.
Recent studies underscore the significance of the capacity factor HPLC in various applications. For example, isobutyric acid exhibits a retention value of 18.3, whereas butyric acid has a retention value of 23.6, highlighting the varying interactions of different compounds with the stationary phase. Furthermore, the selectivity ratio between isobutyric acid and butyric acid stands at 1.29, reflecting the degree of separation attainable under specific conditions. Recognizing the importance of the selectivity ratio is vital for lab managers, as it directly impacts the optimization of chromatographic techniques.
Case studies further illustrate the critical role of the performance metric in enhancing chromatographic separations. Research on the modification of this metric in liquid-solid chromatography demonstrates its influence on resolution, speed, and dilution. By adjusting the distribution coefficient and phase ratio, lab managers can achieve the desired separation outcomes, thereby bolstering the reliability of their analyses. As J.F.K. Huber Eisenbeiss aptly noted,
"The performance ratio is determined by the distribution coefficient and the phase ratio."
In conclusion, a comprehensive understanding of the performance metric is indispensable for lab supervisors aiming to elevate the effectiveness and precision of their chromatographic techniques, ultimately leading to superior analytical outcomes.
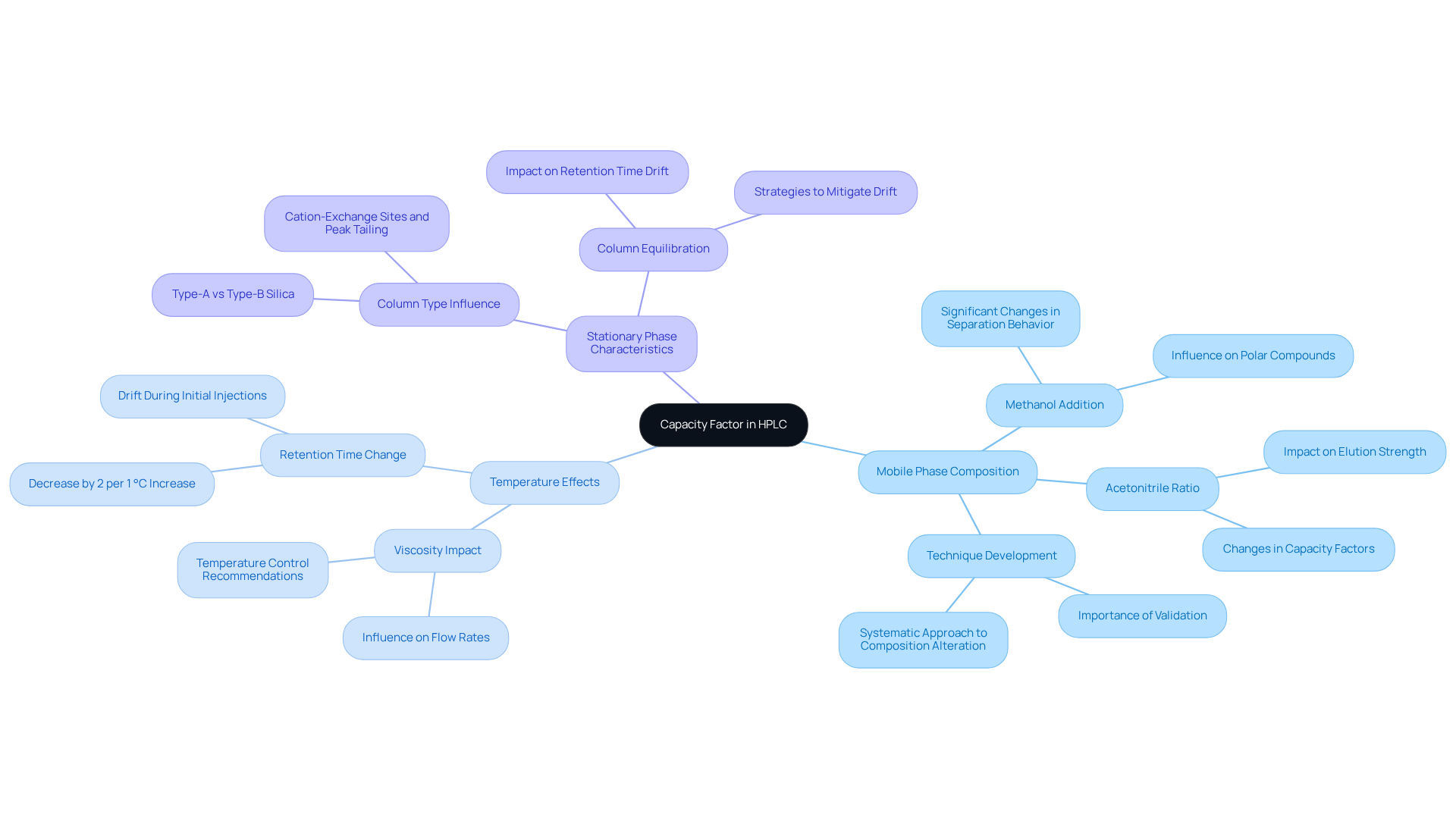
Controlling Factors Affecting Capacity Factor in HPLC
The capacity factor HPLC is critically influenced by several key elements, notably the composition of the mobile phase, temperature, and the characteristics of the stationary phase. Adjusting the solvent composition can lead to significant changes in the interactions between the analyte and the stationary phase, thereby affecting retention times.
For instance, research indicates that the addition of methanol significantly alters the separation behavior, presenting a potential new method for refining HILIC separations of polar compounds. Furthermore, fluctuations in temperature can impact the viscosity of the mobile phase, which in turn influences flow rates and retention times. It has been observed that retention time can decrease by approximately 2% for every 1 °C increase in temperature.
To , it is advisable to utilize a column oven and position the system away from drafts. Additionally, retention time drift may occur during the initial injections of a new column, stabilizing after several uses. Therefore, lab managers must regularly monitor and adjust these parameters, including system dead volume, to ensure optimal performance in their HPLC systems, as the capacity factor HPLC is crucial for achieving consistent retention times and reliable analytical results.
Real-world examples underscore that changes in mobile phase composition, such as the ratio of acetonitrile to methanol, can lead to notable differences in performance metrics, highlighting the importance of thorough technique development and validation in achieving consistent results.
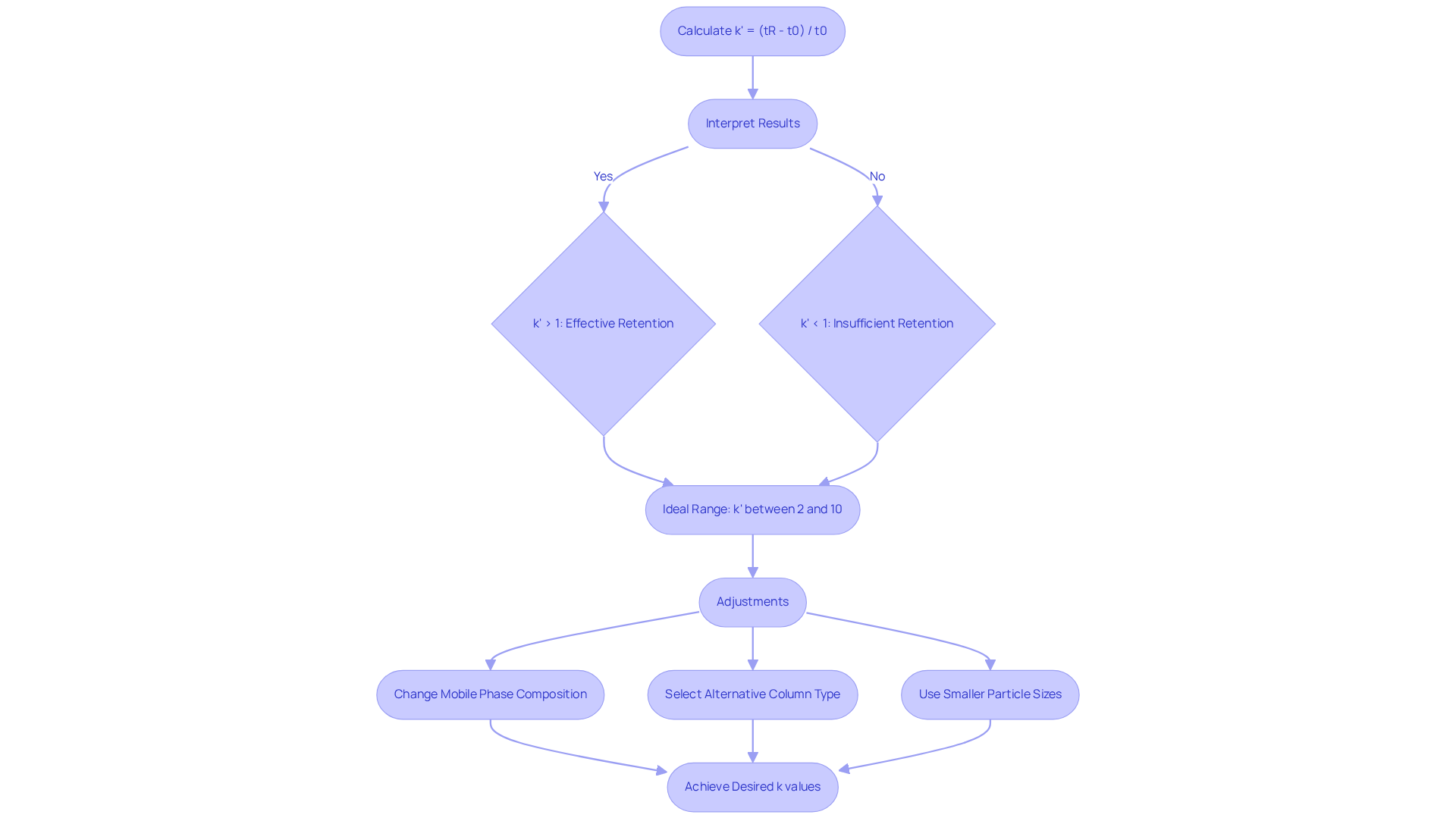
Capacity Factor Formula: How to Calculate and Interpret Results
The performance ratio (k') serves as a crucial parameter in chromatography, defined by the formula: k' = (tR - t0) / t0. Here, tR represents the retention time of the analyte, while t0 denotes the retention time of an unretained peak, also known as void time. Understanding these results is vital; a signifies effective retention of the analyte by the column, whereas values below 1 indicate insufficient retention.
For optimal separation, k' values should ideally range between 2 and 10, ensuring that analytes are neither unretained nor excessively retained, thereby fostering efficient chromatographic techniques. For example, acetic acid displays a retention time of 2.1 minutes, and a moderately acidic compound may show a k' of 1.5 on a C18 column, highlighting potential for improved retention.
Adjustments such as altering the mobile phase composition—significantly affecting the performance ratio—or selecting an alternative column type can facilitate the attainment of desired k values. Furthermore, employing smaller particle sizes in the stationary phase enhances the [capacity factor HPLC](https://linkedin.com/pulse/capacity-factor-hplc-its-significance-fokhrul-abedin-u6puf), which is vital for boosting the overall efficiency of HPLC techniques.
Industry experts emphasize that a thorough understanding of performance metrics is essential for lab managers aiming to refine their analytical procedures and ensure reliable results.
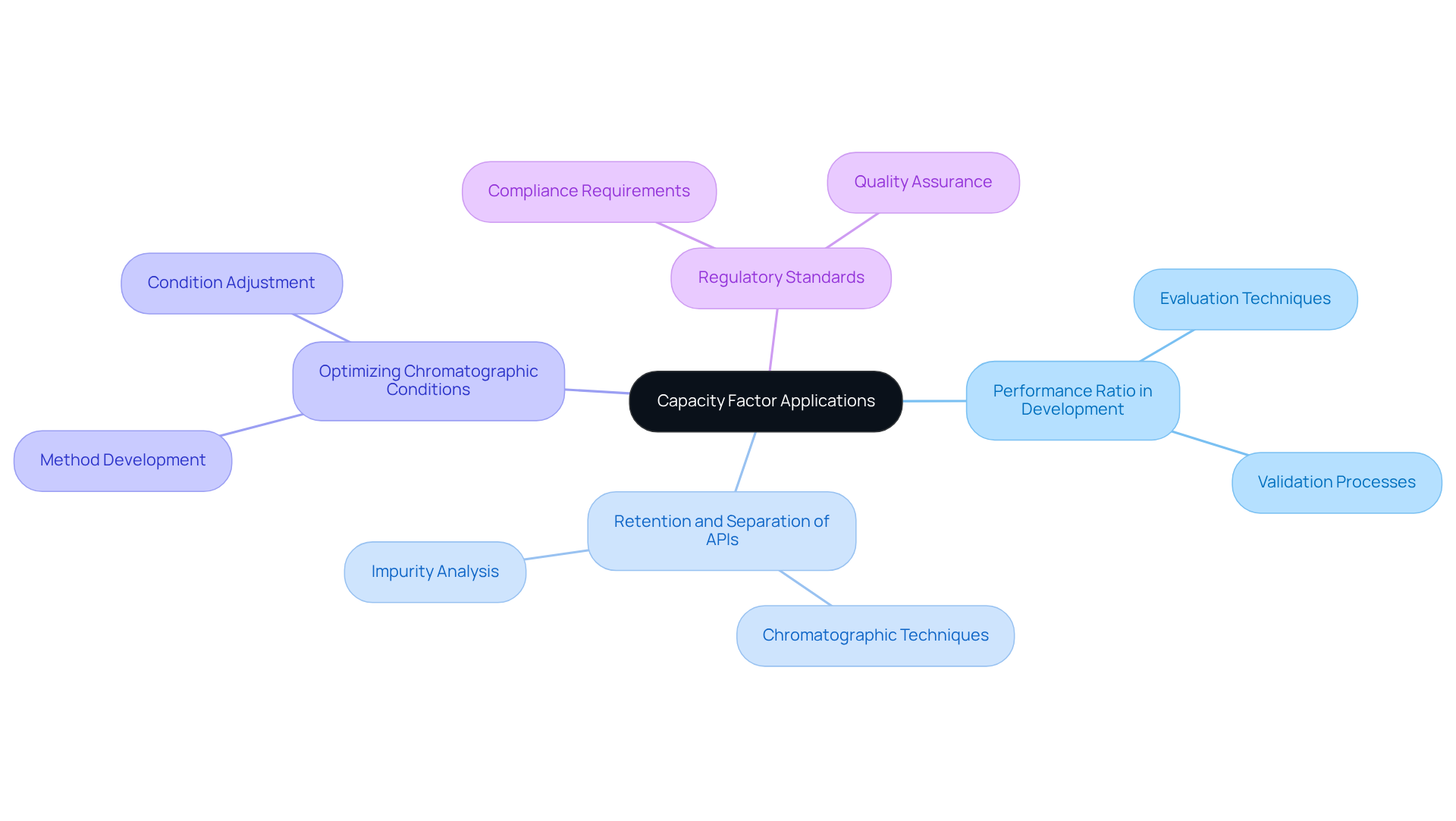
Applications of Capacity Factor in Pharmaceutical and Analytical Chemistry
In the realms of pharmaceutical and analytical chemistry, the performance ratio is pivotal in the development and validation of techniques. This metric is instrumental in evaluating the retention and separation of (APIs) from impurities, thereby ensuring the quality and efficacy of drug formulations. Furthermore, the capacity factor HPLC analysis is essential for optimizing chromatographic conditions, facilitating the efficient separation of complex mixtures. Lab managers can leverage this knowledge to enhance their analytical methodologies while ensuring adherence to regulatory standards. JM Science Inc. stands ready to support these initiatives by offering a comprehensive selection of premium HPLC columns and accessories, which enable precise and reliable chromatographic analysis.
Case Study: Capacity Factor in Action within HPLC Method Development
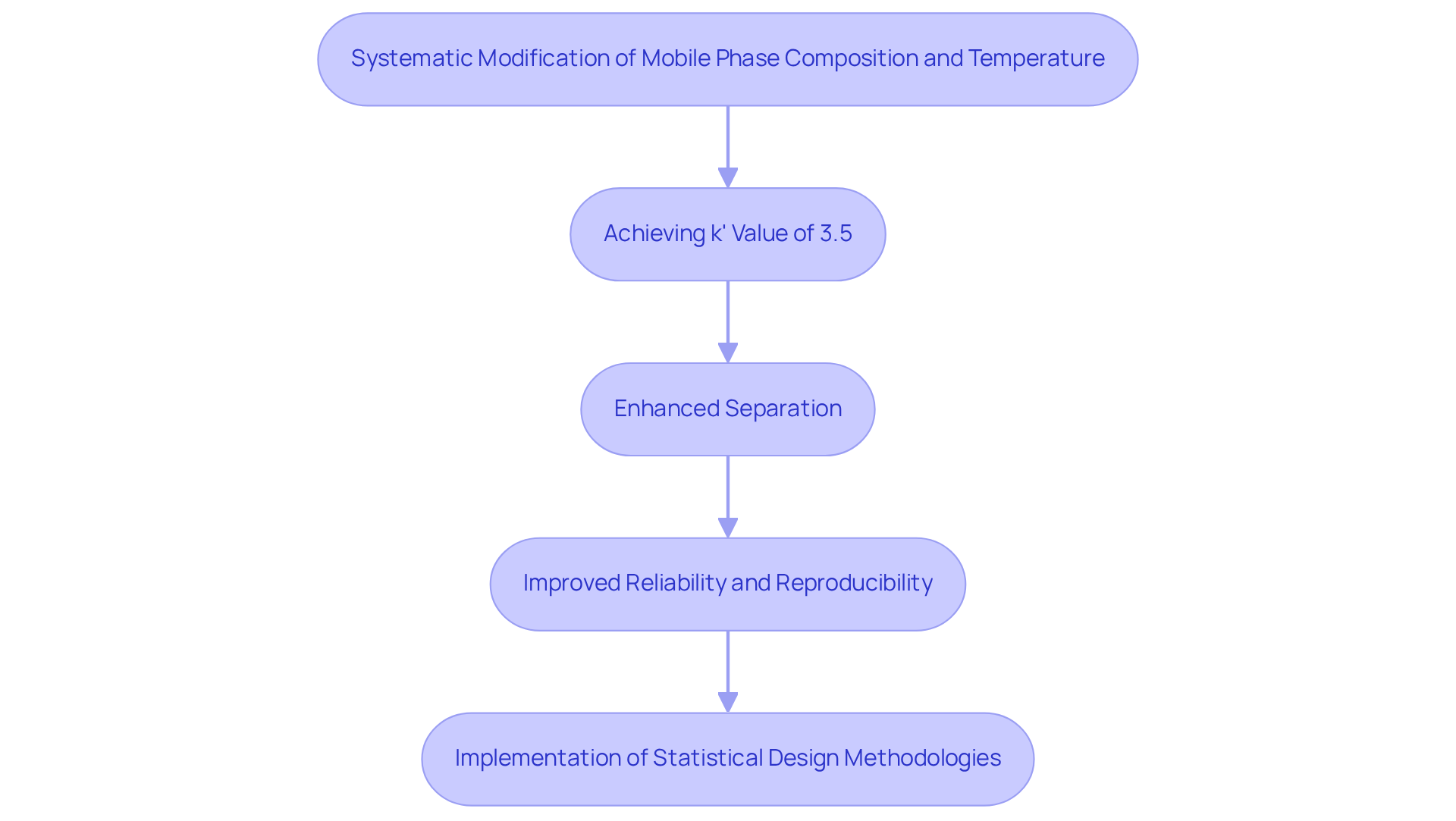
A recent case study conducted in a pharmaceutical laboratory underscores the critical importance of performance variables in the development of techniques for new drug formulations. By systematically modifying the mobile phase composition and temperature, the research team achieved a remarkable improvement in the performance ratio, attaining a k' value of 3.5, widely recognized as a robust indicator of effective separation in the industry. This optimization not only of the drug from its impurities but also significantly bolstered the reliability and reproducibility of the approach. Such real-world applications illustrate how precise adjustments to the performance ratio can yield powerful analytical methods, ultimately facilitating the advancement of high-quality pharmaceuticals.
Moreover, the implementation of statistical design methodologies, including D-optimal designs and response surface methodology, played a pivotal role in this optimization process. The success of this approach reinforces the importance of making informed adjustments to achieve optimal chromatographic performance and highlights the necessity for prequalification to ensure that the process meets standard validation criteria. Readers are encouraged to delve into the case study collection for a deeper understanding of the practical applications of performance metrics, including the capacity factor HPLC, in high-performance liquid chromatography.
Acceptance Criteria for Capacity Factor: Ensuring Quality in HPLC
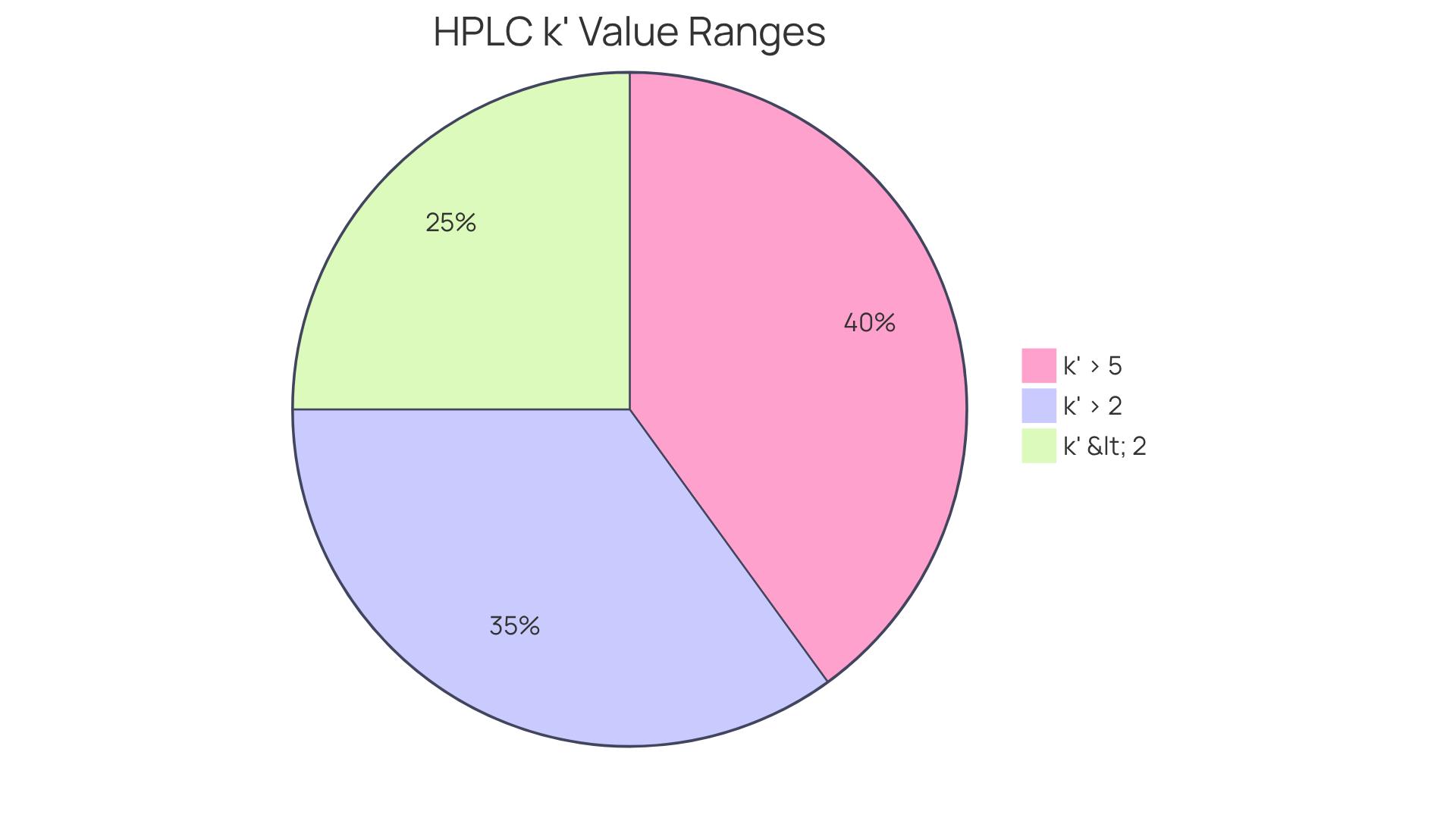
Establishing acceptance criteria for the capacity factor is essential for ensuring the quality and reliability of HPLC techniques. A k' value exceeding 2 is generally recommended for effective separation, while values above 5 are preferred for more complex mixtures. The FDA suggests aiming for a k' value exceeding 2 for . This benchmark allows lab managers to assess the efficiency of their chromatographic techniques and ensure compliance with industry standards. Regular reviews and updates of these criteria are crucial for sustaining high-quality analytical outcomes.
Studies have demonstrated that maintaining a k value within these recommended ranges significantly enhances technique robustness and reproducibility, which are vital for achieving consistent results in pharmaceutical applications. Moreover, industry leaders emphasize that stringent quality assurance practices related to k values—such as monitoring intra-analytical QA components and ensuring lot-to-lot verification—not only enhance validation processes but also aid in meeting regulatory standards. This ultimately results in more dependable data in clinical environments.
As noted by the European Federation of Clinical Chemistry and Laboratory Medicine, maintaining stringent quality control measures is imperative for the integrity of analytical results. By prioritizing these acceptance criteria, laboratories can significantly improve their operational efficiency and maintain a high capacity factor HPLC to uphold the highest standards of analytical excellence.
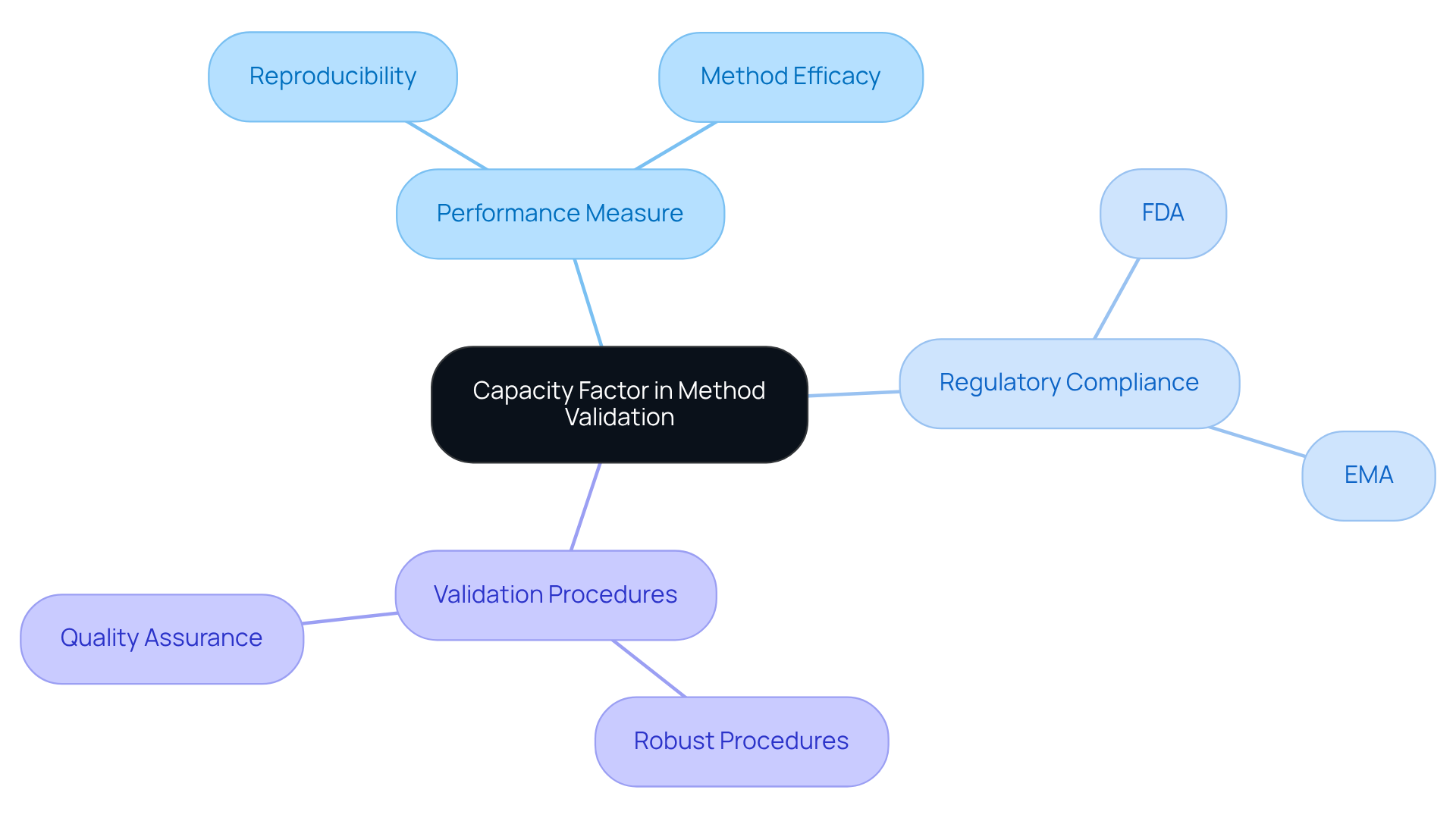
Capacity Factor in Method Validation: Key Considerations for Compliance
In the realm of procedure validation, the performance measure stands as a crucial parameter that demands thorough evaluation to ensure compliance with regulatory standards. Laboratories must demonstrate that their methods consistently yield reproducible results, with the performance measure serving as a vital indicator of method efficacy.
To meet the stringent requirements set forth by regulatory bodies such as the FDA and EMA, lab supervisors should establish robust that integrate performance evaluations. This approach not only satisfies regulatory expectations but also reinforces the laboratory's commitment to quality and reliability.
Linking Capacity Factor and Separation Efficiency in HPLC Techniques
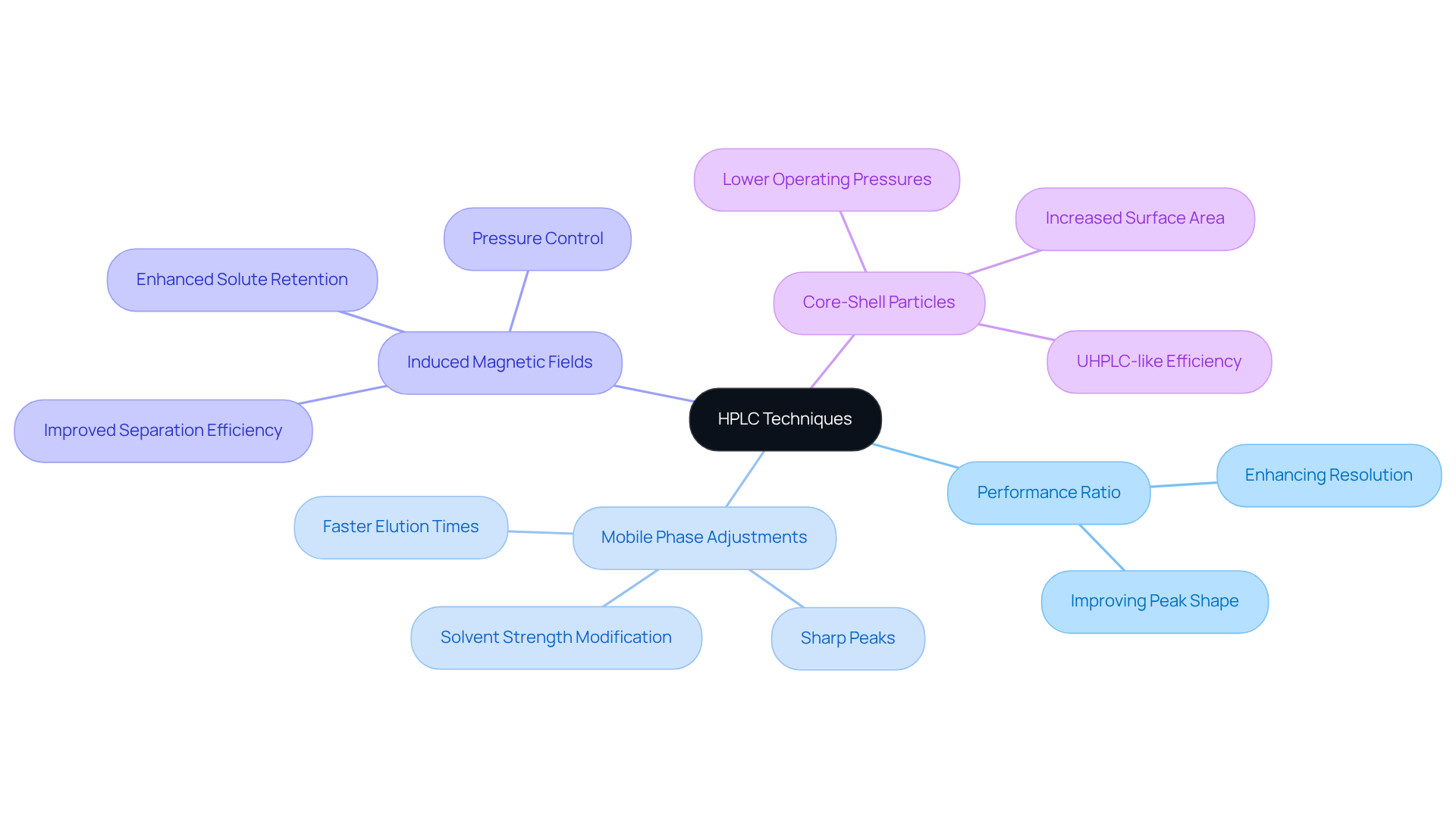
The performance ratio is pivotal in evaluating separation efficiency within HPLC methods. A refined performance metric not only enhances resolution but also improves peak shape—both are essential for the effective separation of analytes. Lab managers can significantly elevate chromatographic efficiency by strategically fine-tuning the capacity through adjustments in mobile phase composition and flow rates. For example, modifying the solvent strength can result in faster elution times and sharper peaks, which are crucial for accurate analysis. Furthermore, the application of induced magnetic fields (MF) can enhance solute retention and separation efficiency, offering an innovative approach to optimizing chromatographic conditions.
Real-world applications illustrate that laboratories utilizing core-shell particles have achieved remarkable advancements in separation efficiency. These enhancements allow for operations at lower pressures while maintaining high performance. This optimization is vital for the development of techniques that ensure a high capacity factor HPLC to meet the rigorous demands of modern analytical chemistry. It ensures that laboratories can comply with regulatory standards and improve their analytical capabilities, all while ensuring product purity and stability.
Challenges and Solutions: Navigating Capacity Factor Issues in HPLC
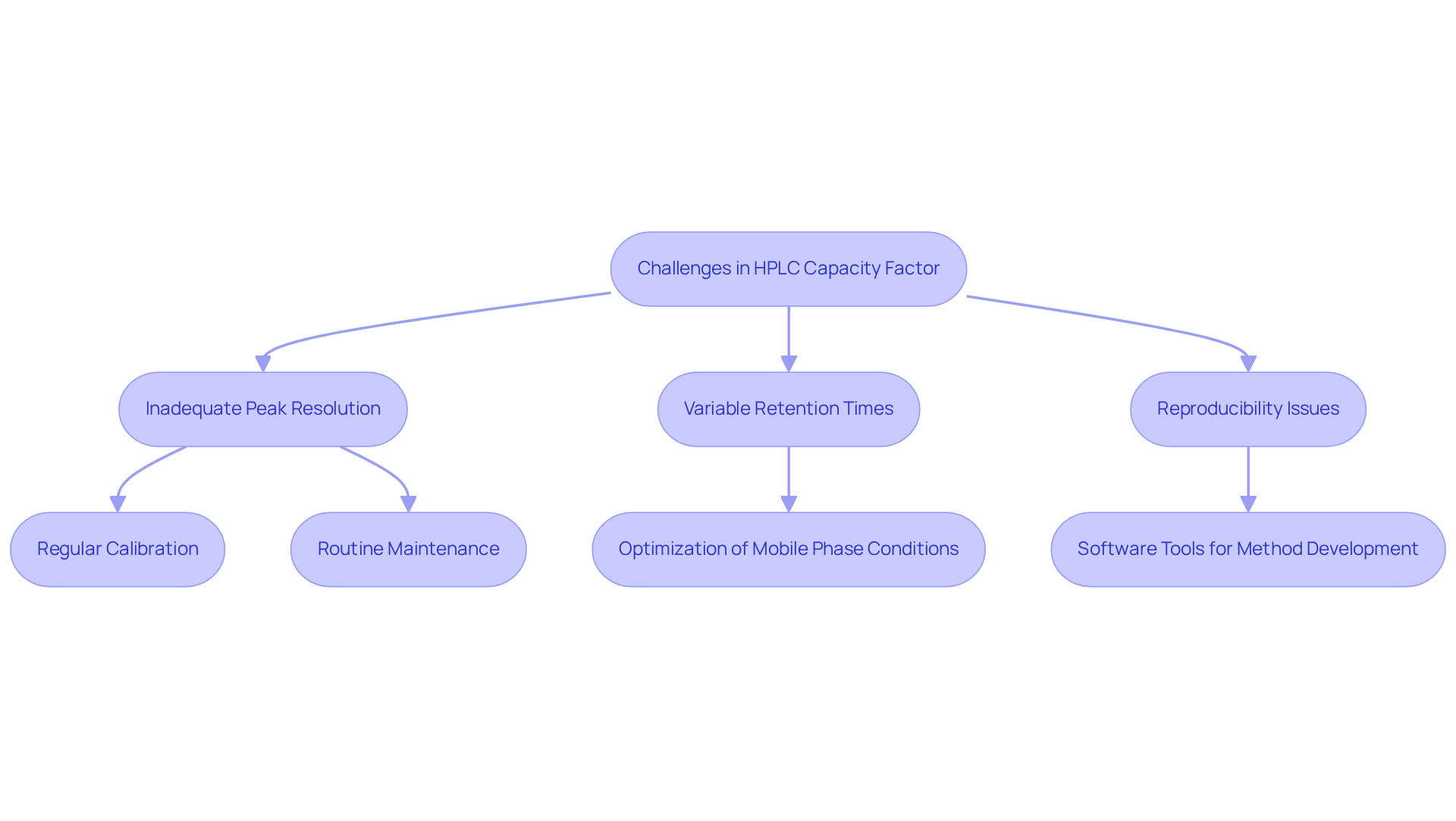
Challenges related to the capacity factor HPLC in high-performance liquid chromatography frequently manifest as inadequate peak resolution, variable retention times, and reproducibility issues in techniques. To effectively address these challenges, lab managers can implement several .
- Regular Calibration: Consistent calibration of instruments is paramount to maintaining accuracy and reliability in measurements.
- Optimization of Mobile Phase Conditions: Tailoring the composition and flow rate of the mobile phase can significantly enhance separation efficiency and capacity factors.
- Routine Maintenance: Regular maintenance of chromatography systems is essential for ensuring optimal performance and prolonging equipment lifespan. For instance, the Tunnel Series Thermoelectric Cooler Assembly is specifically designed for HPLC applications, providing effective temperature regulation that enhances procedure reproducibility and peak resolution.
- Software Tools for Method Development: Utilizing advanced software can streamline the identification of optimal conditions, facilitating the achievement of desired performance levels.
Industry leaders have reported success rates exceeding 90% when these solutions are implemented, underscoring their effectiveness in improving analytical performance. Innovations in thermoelectric cooling technology, as highlighted by Tark Thermal Solutions, play a vital role in stabilizing temperatures, thereby bolstering method reproducibility and peak resolution. By proactively tackling capacity factor HPLC challenges, laboratories can markedly enhance their analytical capabilities and ensure consistent, reliable results.
Conclusion
The capacity factor stands as a pivotal metric in high-performance liquid chromatography (HPLC), acting as a crucial indicator of an analyte's retention and separation efficiency. Grasping this parameter empowers lab managers to refine their methodologies, thereby ensuring enhanced accuracy and reproducibility in analytical results. By harnessing advanced technologies and techniques, laboratories can optimize their chromatographic processes, ultimately yielding more dependable outcomes across various applications.
Key insights presented throughout this article underscore the significance of the capacity factor in optimizing chromatographic conditions. This includes managing influencing factors such as mobile phase composition and temperature, as well as adhering to established industry standards. The imperative of setting acceptance criteria for capacity factors is also highlighted, ensuring that laboratories uphold high-quality analytical practices while achieving effective separations.
In conclusion, the capacity factor transcends being merely a numerical value; it encapsulates the quality and reliability of analytical results in HPLC. As laboratories endeavor to meet the evolving demands of pharmaceutical and analytical chemistry, prioritizing the understanding and optimization of this metric is essential. By embracing innovations, implementing best practices, and persistently refining techniques, lab managers can significantly bolster their analytical capabilities and contribute to the advancement of high-quality research and development in the field.




