Overview
The article underscores the pivotal role of KCl solution in ensuring the accuracy and reliability of pH measurements within laboratory environments. A 3 M KCl solution is not merely beneficial; it is essential for maintaining the hydration and stability of pH sensors. This stability significantly enhances their responsiveness and precision. As a result, consistent measurement outcomes are supported across a variety of applications, reinforcing the necessity of high-quality scientific instruments in laboratory settings.
Introduction
In the intricate realm of laboratory practices, the significance of KCl solutions in ensuring accurate pH measurements is paramount. These solutions are not merely supplementary to the scientific process; they are essential for upholding the integrity and reliability of pH electrodes. By establishing a stable ionic environment, KCl solutions enhance both the responsiveness and longevity of these critical instruments, rendering them indispensable for achieving precise experimental outcomes.
As laboratories increasingly prioritize accuracy and efficiency, grasping the nuances of KCl solutions—from optimal storage conditions to regulatory compliance—becomes crucial. This article examines the vital role of KCl solutions in laboratory settings, investigating best practices for handling, environmental considerations, and the cost implications associated with their use.
JM Science 3 M KCl Solution: Essential for pH Electrode Storage
The 3 M KCl solution from JM Science is crucial for the effective storage of pH sensors. It prevents the glass bulb from drying out—a vital factor in preserving the sensor's sensitivity to hydrogen ions. By ensuring consistent hydration, this mixture significantly enhances the sensor's responsiveness and precision, thereby improving the reliability of pH measurements across diverse laboratory applications.
Indeed, the optimal storage method for pH sensors is a KCl solution with a 3 M concentration, guaranteeing their efficient operation and the delivery of reliable, accurate readings. Furthermore, the use of KCl mixtures in electrode maintenance is supported by professional insights, underscoring their critical role in prolonging the longevity and effectiveness of these essential instruments.
As noted, 'Filtering might eliminate some impurities, but it may not suffice for precise readings,' which highlights the necessity of utilizing a KCl solution for dependable pH measurements. This commitment to quality storage solutions aligns with JM Science's dedication to advancing scientific practices, not only facilitating accurate results but also reinforcing the company's position in enhancing efficiency within research facilities.
Role of KCl Solution in pH Measurement Accuracy
KCl solution mixtures are essential for ensuring the accuracy of pH measurements in laboratory environments. The potassium ions present in KCl mixtures establish a stable reference potential within the pH electrode, significantly reducing drift and fluctuations in readings. This stability is vital for delivering consistent and reliable results, as erratic readings can jeopardize experimental outcomes. Research indicates that the addition of cellobiose can further enhance this stability, maximizing the inhibition of pH changes by approximately 0.62 units. This underscores the necessity of maintaining a stable ionic environment for precise measurements.
Expert opinions emphasize that slight adjustments towards acidic or basic pH levels can facilitate the formation of larger, more stable flocs. This not only simplifies their removal but also enhances measurement reliability. Such adjustments are particularly relevant in the context of KCl mixtures, as the stability they provide can be further augmented through these modifications.
In a case study titled "Influence of pH and Blend Ratios on Complex Coacervation in Composite Hydrogels," researchers explored the effects of pH and blend ratios on gelation properties. The findings revealed that lower pH values enhance gel strength and microstructural properties, laying the groundwork for the development of innovative hydrogels. This illustrates how a KCl solution is pivotal in stabilizing pH measurements, thereby influencing the outcomes of diverse scientific applications.
Moreover, Michael T Pope noted that "formal reduction potentials for the initial one-electron reductions vary with the ionic charge in a manner consistent with a simple electrostatic model for the electron affinities of these approximately spherical polyanions." This insight underscores the critical role of potassium ions in stabilizing electrode readings, as their behavior is intricately linked to the electrostatic interactions within the mixture.
In conclusion, the function of the KCl solution in preserving the precision of pH measurements is paramount. For laboratories striving to achieve accurate and dependable results, the utilization of a KCl solution is not merely advantageous but essential.
Optimal Storage Conditions for KCl Solutions
To maximize the longevity and effectiveness of KCl mixtures, it is essential to store them in a cool, dark environment, away from direct sunlight and extreme temperatures. Tightly sealed containers are critical to prevent evaporation and contamination, which can compromise the integrity of the mixture. Regular inspections for signs of precipitation or degradation are advisable to ensure optimal performance.
Research indicates that low conductivity liquids, defined as having less than 100 µS/cm, can be adversely affected by improper storage conditions, leading to potential inaccuracies in pH measurements. Furthermore, maintaining high-quality components through appropriate upkeep, including the use of a KCl solution, significantly enhances the precision and reliability of titration procedures.
As Dewan Rafi, Senior Executive of Quality Assurances at Tropical Knitex Ltd., states, "Storing the device in KCl prevents these imbalances by ensuring that both the internal and external environments of the device remain stable."
Expert recommendations suggest that KCl preparations should be kept at temperatures ranging from 4°C to 25°C to maintain their effectiveness. Additionally, monitoring the mV value in a pH 7 buffer can indicate the lifespan of the sensor; readings greater than +/- 30mV suggest it may require replacement.
By adhering to these best practices, laboratories can ensure that their KCl solution preparations remain stable and reliable for accurate pH measurements.
Impact of KCl Concentration on Electrode Performance
The concentration of KCl solution in the storage solution is paramount to the performance of pH sensors. A KCl solution with a concentration of 3 M is widely recommended, as it strikes an optimal balance between conductivity and stability. This level of ionic strength significantly enhances the sensor's response time and precision, ensuring reliable measurements. Conversely, higher concentrations may result in excessive ionic strength, potentially compromising sensor performance. Lower concentrations, on the other hand, may fail to provide sufficient ionic strength, leading to unstable readings. Therefore, it is essential to maintain the recommended KCl solution concentration to achieve optimal electrode response and ensure accurate pH measurements.
Furthermore, KCl solution mixtures do not alter the acidity or alkalinity of the liquid being examined; instead, they create a stable electrical environment conducive to reliable pH measurements. Adjusting the pH during coagulation processes can further amplify the effectiveness of coagulants, resulting in larger and more stable flocs that are easier to remove in subsequent treatment steps.
As noted by Edward L., a wastewater and sludge treatment solution provider, 'Adjusting the pH during coagulation enhances the effectiveness of the coagulants.' This underscores the importance of precise KCl solution concentrations, not only for sensor performance but also for broader applications in laboratory settings.
For terminal maintenance, it is also beneficial to note that salt crystals can be effectively removed by soaking in 0.1M HCl for up to 20 minutes, thereby ensuring optimal performance. Moreover, understanding the operational principles of a glass pH sensor can streamline the measurement process, facilitating the implementation of effective practices for lab managers.
Compatibility of KCl Solutions with pH Electrodes
KCl mixtures are known for their compatibility with a wide range of pH sensors, including both glass and combination types. It's crucial to adhere to the manufacturer's guidelines, as certain components may necessitate specific storage methods to guarantee optimal performance. Using an inappropriate method can result in damage or lead to inaccurate measurements.
For instance, gel-filled sensors may experience a decline in performance when exposed to overly concentrated KCl mixtures, potentially causing blockage of the junction and subsequent measurement inaccuracies.
As Rajesh Das, Production Executive at Sun Pharma, states, 'By keeping the pH probe in a KCl solution, you maintain its precision, avoid contamination, and prolong its lifespan, ensuring it operates efficiently over time.'
Utilizing the correct KCl solution not only preserves the integrity of the electrodes but also enhances the reliability of pH readings.
KCl Solutions and Laboratory Safety Protocols
Following safety protocols in the lab is essential when managing the KCl solution mixtures. Personal protective equipment (PPE), including gloves, goggles, and lab coats, is critical to prevent skin and eye contact. All waste, particularly sharp items like scalpels and needles, must be disposed of in designated biohazard containers to ensure safety and compliance with regulations. A case study on handling biological materials safely underscores the necessity of using gloves and sterilizing work surfaces, significantly reducing contamination risks associated with KCl solution. Furthermore, expert insights emphasize that technical staff must be trained in these protocols to minimize exposure and environmental impact.
In the event of a spill, immediate cleanup procedures must be implemented. To maintain a safe working environment, it is recommended that work surfaces be cleaned at the end of each day, with waste disposed of in lined biohazard bins to prevent spills. As noted by Ju Lin Tan, PhD, "Please ensure that you line the bins with two layers of biohazard bags to avoid spilling of biological material." Adhering to these guidelines not only safeguards research staff but also enhances the overall integrity of scientific and healthcare practices, particularly in the context of the KCl solution.
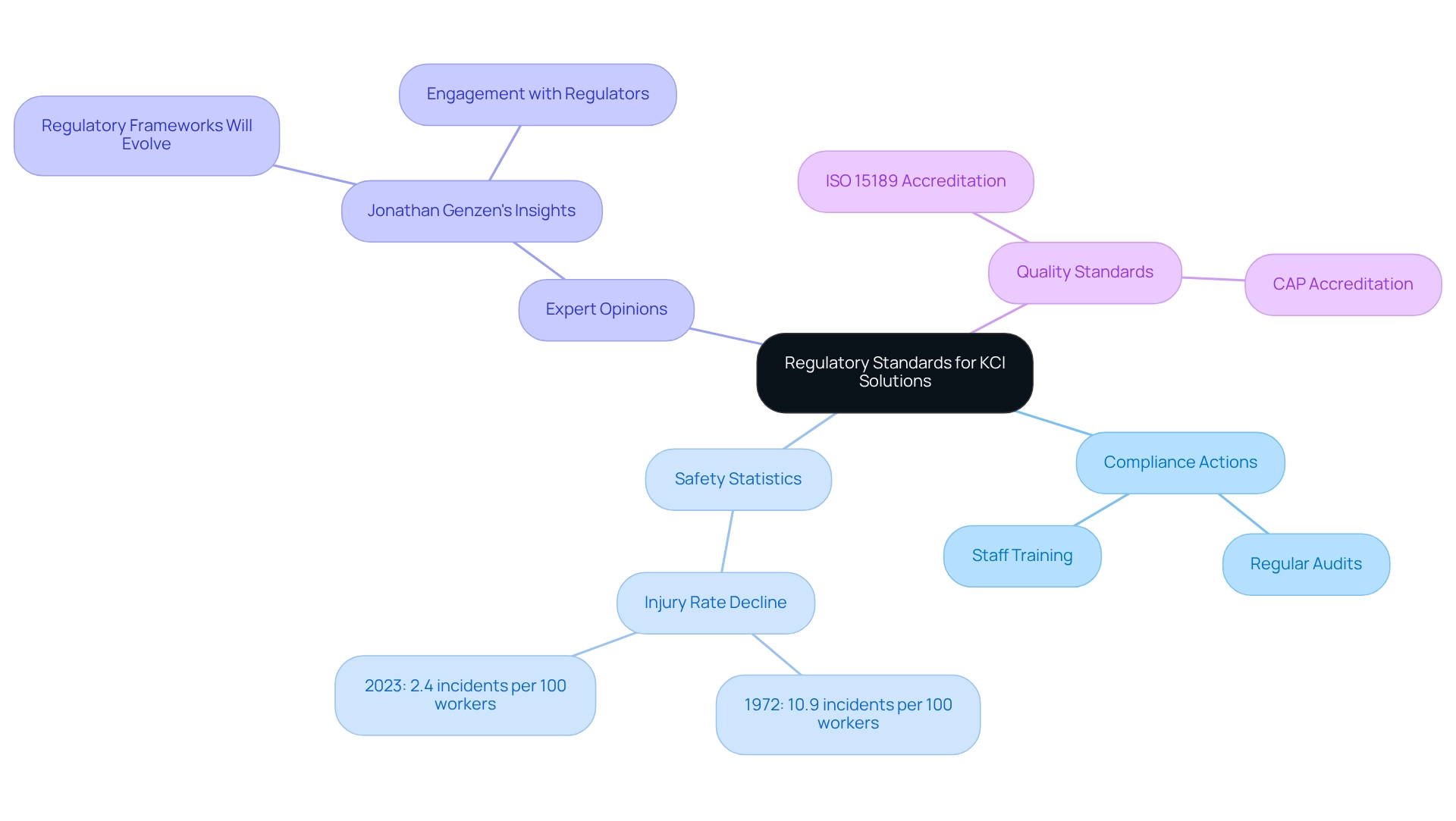
Regulatory Standards for KCl Solutions in Laboratories
Laboratories utilizing KCl solution must adhere to stringent regulatory standards set forth by the Occupational Safety and Health Administration (OSHA) and the Environmental Protection Agency (EPA). These regulations outline essential protocols for the safe handling, storage, and disposal of chemical substances, ensuring the protection of personnel and the environment. Compliance transcends mere legal obligation; it is vital for maintaining a safe working environment and minimizing risks associated with chemical exposure.
To uphold these standards, facilities should:
- Conduct regular audits.
- Provide extensive training sessions for all staff.
This proactive approach ensures that team members are well-informed about the latest regulations and best practices, fostering a culture of safety and compliance. For instance, OSHA statistics reveal a significant decline in worker injuries and illnesses, plummeting from 10.9 incidents per 100 workers in 1972 to just 2.4 incidents per 100 workers in 2023, underscoring the effectiveness of stringent safety protocols.
Moreover, expert opinions underscore the importance of continuous education regarding OSHA and EPA guidelines for the handling and disposal of the KCl solution. As Jonathan Genzen, MD, PhD, MBA, ARUP's chief medical officer, stated, "We recognize that regulatory frameworks will evolve, and we will continue to engage with those who create regulations to best serve our clients and patients." By remaining informed about regulatory changes and participating in ongoing training, facilities can enhance their operational safety and efficiency, ultimately leading to improved outcomes in research and clinical environments.
Furthermore, facilities should strive to achieve high-quality standards, such as those recognized by ISO 15189 and CAP, which serve as benchmarks for compliance and quality assurance. Instances of facilities successfully implementing these regulatory standards can provide invaluable insights for lab managers. Additionally, ARUP's support for clinical labs and interaction with regulatory agencies highlights the importance of active involvement in regulatory conversations, ensuring that facilities remain informed and compliant in an ever-evolving landscape.
Environmental Considerations for KCl Solution Use
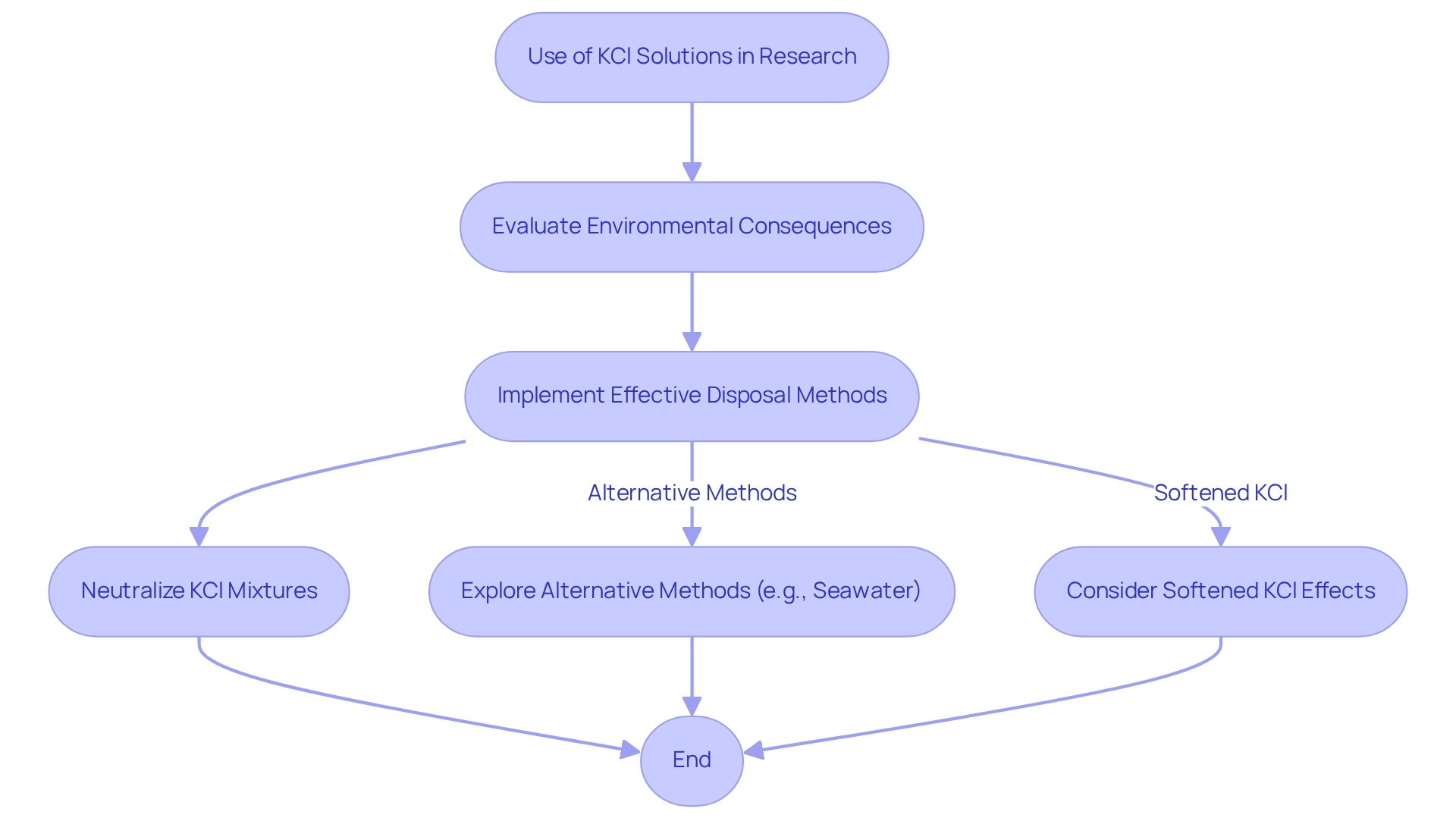
The environmental consequences of employing KCl preparations in research facilities necessitate thorough evaluation and strict adherence to waste management guidelines. To mitigate the risk of contaminating water sources, facilities must implement effective disposal methods, including the neutralization of KCl mixtures prior to disposal. This practice not only protects the environment but also complies with regulatory standards designed to safeguard aquatic ecosystems.
Moreover, reducing the application of KCl mixtures is advisable, prompting facilities to explore alternative methods that yield similar results without adverse environmental effects. For example, recent studies have underscored the potential of seawater as a regenerant in anion exchange processes, showcasing its effectiveness while significantly lowering environmental impacts. This approach highlights the critical role of innovation in waste management practices and aligns with findings that seawater can effectively regenerate anion resins, thereby enhancing both efficiency and sustainability.
Furthermore, softened potassium chloride mixtures pose no threat to plants, as potassium is an essential nutrient. Allowing the water to stand for one to five days can further diminish chlorine levels, thereby enhancing its environmental safety. Statistics reveal that ion exchange processes can achieve remarkable contaminant removal rates, with studies indicating over 60% removal of natural organic matter (NOM) and more than 80% of inorganic ions. This underscores the effectiveness of proper waste management protocols in achieving substantial contaminant removal.
Incorporating specialist insights on waste management procedures for the KCl solution mixtures can significantly enhance laboratory practices. As Philip C. Singer noted, "This work provides an improved understanding of the interactions between natural organic matter (NOM), inorganic anions, and anion exchange resins, and should result in more effective applications of ion exchange for the removal of NOM in the treatment of drinking water." By emphasizing ecological considerations in the disposal of KCl mixtures, facilities can contribute to sustainable practices while ensuring compliance with environmental regulations.
Cost Implications of KCl Solutions in Laboratory Operations
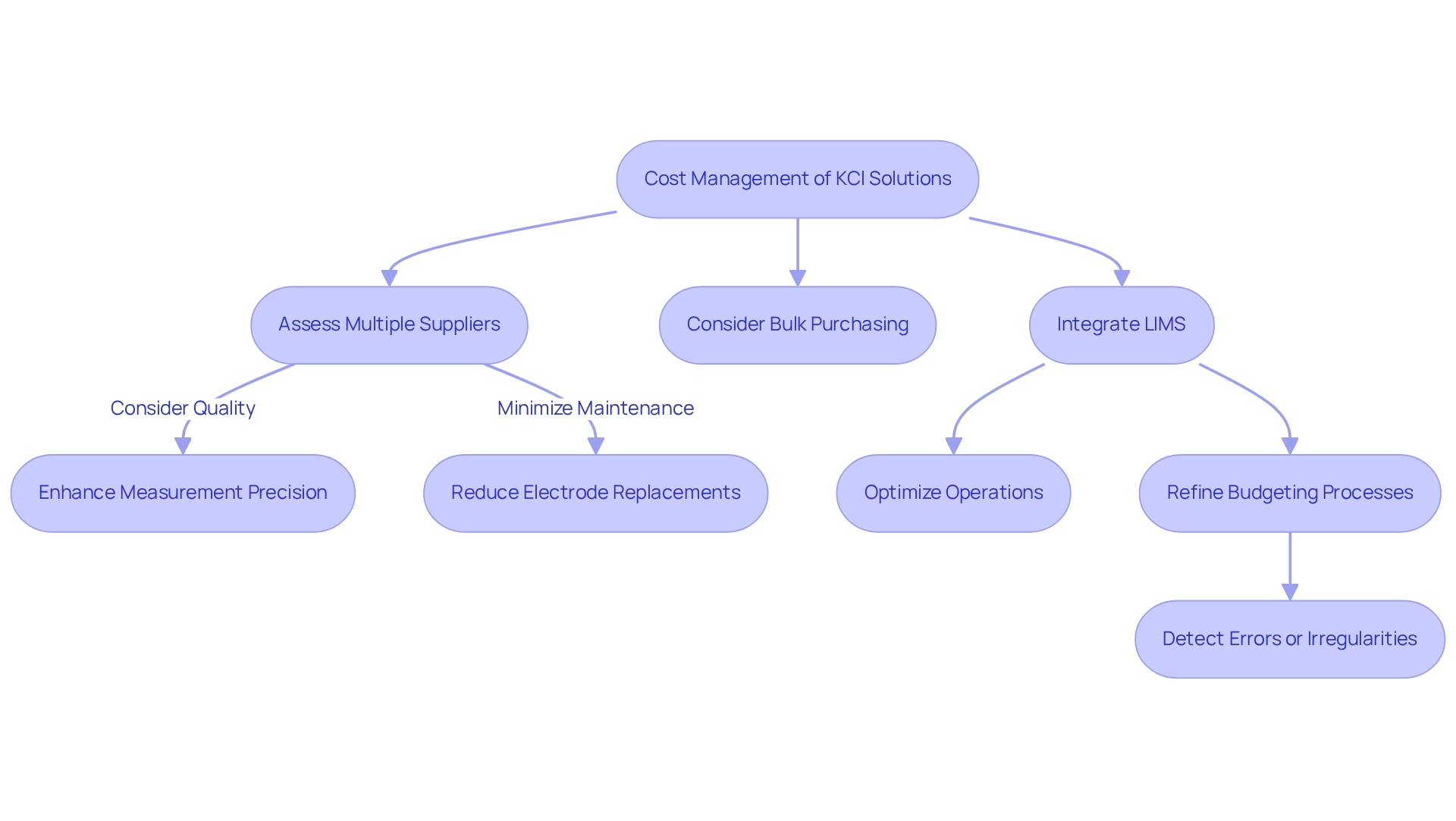
The pricing of the KCl solution mixtures is influenced by factors such as concentration and supplier selection, underscoring the necessity for facilities to meticulously strategize their budgets for pH measurement tasks. While KCl solution mixtures are typically low-cost, expenses can accumulate significantly in high-volume environments. To manage these costs effectively, it is advisable to:
- Assess multiple suppliers
- Consider bulk purchasing options, which can yield considerable savings
Furthermore, investing in high-quality KCl solution not only enhances measurement precision but also reduces long-term expenses by minimizing the frequency of electrode replacements and maintenance needs.
Integrating an advanced Laboratory Information Management System (LIMS) can further optimize operations and refine budgeting processes. As emphasized by Clinisys, "Leveraging an advanced LIMS allows labs to streamline operations, adapt to market changes, and position themselves for sustainable growth in a competitive landscape." The Clinisys Contract Services Laboratory™ case study illustrates how proficient management via LIMS can result in cost savings and improved operational efficiency. By adopting strategic budgeting practices and employing tools like budget analysis to detect errors or irregularities, facilities can enhance their procurement processes and ensure effective resource allocation.
Best Practices for Handling KCl Solutions in the Lab
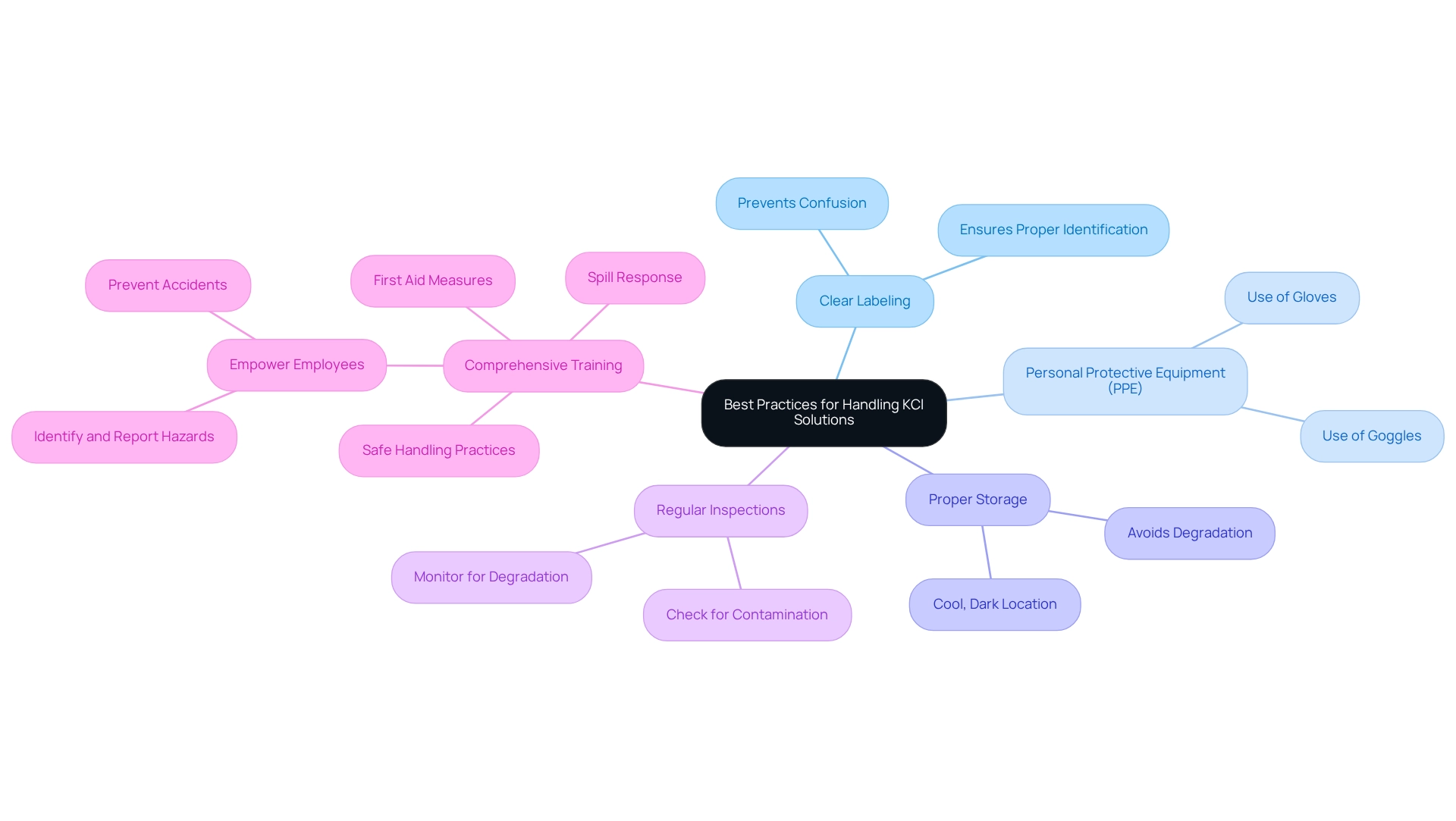
To ensure the safe and effective handling of KCl solution, laboratories must implement best practices that are crucial for maintaining safety and integrity.
- Clear Labeling: It is imperative to label containers clearly, preventing confusion and ensuring proper identification of contents.
- Personal Protective Equipment (PPE): Appropriate PPE, such as gloves and goggles, should always be utilized to protect personnel from potential exposure.
- Proper Storage: KCl mixtures must be stored in a cool, dark location to ensure their stability and avoid degradation.
- Regular Inspections: Routine checks for signs of contamination or degradation are essential to uphold the integrity of the mixtures.
- Comprehensive Training: Training for all personnel on proper handling techniques and emergency procedures related to KCl mixtures is non-negotiable. This training should encompass safe handling practices, spill response, and first aid measures. As Robert Bouchard, a rack expert, states, "Train your employees on rack safety and they’ll identify and report the damage. Empower them to report damage and you’ll avoid accidents."
By adhering to these practices, facilities can significantly enhance safety and maintain the integrity of their pH measurement processes. Moreover, fostering a culture that prioritizes safety is essential; it must be reflected in all organizational aspects, from leadership to daily operations. Empowering employees through training not only equips them to handle KCl solutions safely but also encourages them to identify and report potential hazards, which helps prevent accidents and ensures a safer work environment. This dedication to safety should resonate throughout the organization, reinforcing the importance of a safety culture in research environments. Furthermore, as demonstrated by KPM Analytics, innovation and safety practices can drive improvements in laboratory environments, underscoring the critical nature of these principles.
Conclusion
The pivotal role of KCl solutions in laboratory settings is paramount. They ensure the accurate storage of pH electrodes and maintain the stability necessary for precise pH measurements. KCl solutions are integral to the reliability of laboratory operations. The recommended concentration of 3 M KCl strikes a balance that enhances electrode performance, while adherence to optimal storage conditions and safety protocols guarantees the longevity and effectiveness of these solutions.
Moreover, understanding the compatibility of KCl solutions with various types of pH electrodes, alongside regulatory standards, emphasizes the importance of informed practices in laboratory management. By prioritizing proper handling, regular inspections, and compliance with environmental considerations, laboratories can mitigate risks and contribute to sustainable practices.
In conclusion, investing in high-quality KCl solutions and implementing best practices not only enhances measurement accuracy but also reduces long-term operational costs. As laboratories continue to evolve, embracing these principles will foster a culture of safety, efficiency, and reliability, ultimately leading to better experimental outcomes and advancements in scientific research.




