Overview
This article delineates ten pivotal benefits of employing monolithic column HPLC in pharmaceutical laboratories. Among these, enhanced efficiency, cost-effectiveness, improved resolution, and versatility for diverse applications stand out. Evidence substantiating these advantages includes:
- Faster analysis times
- Reduced solvent usage
- Superior separation capabilities
- User-friendly operation
Collectively, these factors significantly elevate productivity and ensure compliance with industry standards in drug development and testing.
Introduction
In the rapidly evolving landscape of pharmaceutical research and development, the demand for precision and efficiency in analytical processes is paramount. JM Science Inc. leads the charge, providing a comprehensive suite of high-performance liquid chromatography (HPLC) solutions meticulously designed to meet the stringent requirements of the industry. Their innovative tools, ranging from advanced HPLC columns to sophisticated detectors, not only guarantee reliability but also significantly enhance productivity in drug testing and development.
As laboratories endeavor to keep pace with the complexities of modern pharmaceuticals, the integration of cutting-edge technologies, such as monolithic columns, emerges as a game-changer. These advancements promise faster analyses, reduced costs, and improved resolution.
This article explores the multifaceted benefits of these technologies, underscoring how they empower pharmaceutical labs to optimize operations while adhering to rigorous regulatory standards.
JM Science HPLC Solutions: Precision Instruments for Pharmaceutical Applications
JM Science Inc. is at the forefront of solutions in high-performance liquid chromatography (HPLC), particularly with their meticulously designed monolithic column HPLC for drug-related applications. Their impressive product lineup features cutting-edge HPLC columns, including monolithic column HPLC, detectors, and essential accessories that guarantee precision and reliability throughout analytical processes. Among these, the AQ-300 Coulometric and AQV-300 Volumetric emerge as indispensable tools for drug and medicine testing, fully compliant with the stringent standards set by the Japanese Pharmacopoeia.
Collaborating with industry leaders such as Agilent Technologies and Thermo Fisher Scientific, JM Science ensures that its instruments meet the rigorous standards of research and quality control. These advanced tools, such as the monolithic column HPLC, are expertly engineered to facilitate precise separation and analysis of complex drug compounds, making them vital assets in drug development and testing environments.
Moreover, JM Science's unwavering commitment to ethical standards in data collection and analysis reinforces the quality and reliability of their products, solidifying their esteemed reputation within the industry. By prioritizing excellence, JM Science empowers researchers and laboratories to achieve unparalleled results in their scientific endeavors.
Enhanced Efficiency: Faster Analysis with Monolithic Columns
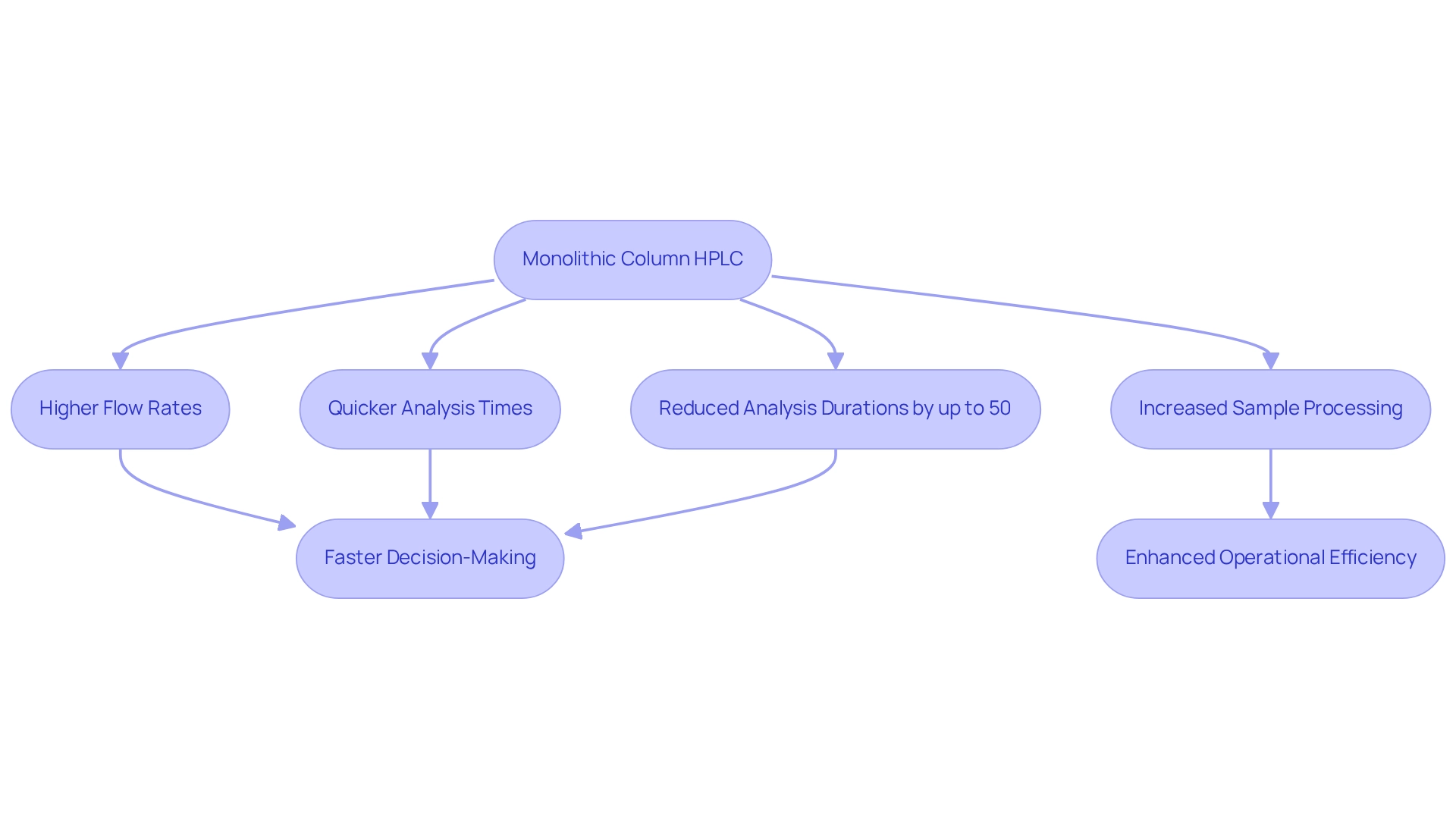
Monolithic column HPLC structures are designed to support high flow rates, leading to significantly quicker analysis times compared to traditional packed systems. This enhanced efficiency is especially advantageous in drug testing laboratories, where timely results are paramount. Research indicates that unified pillars can achieve separations in a fraction of the time required by conventional techniques, enabling laboratories to process a greater volume of samples without sacrificing quality. This capability not only amplifies productivity but also aligns with the .
For instance, studies have demonstrated that the implementation of solid pillars can reduce average analysis durations by up to 50%, facilitating faster decision-making in critical drug manufacturing processes. Furthermore, preparative and semi-preparative silica-based structures are available for the purification and isolation of active compounds from raw materials in natural medicine and pharmaceuticals.
Moreover, case studies highlight how these structures adeptly address the challenges posed by complex biological matrices, maintaining separation efficiency while minimizing clogging and backpressure. As Tohru Ikegami noted, recent advancements in monolithic column HPLC have improved their chromatographic characteristics, establishing them as invaluable resources in modern laboratories. Consequently, drug companies can enhance their operational efficiency and responsiveness to market demands.
Cost-Effectiveness: Reduced Solvent and Waste Usage
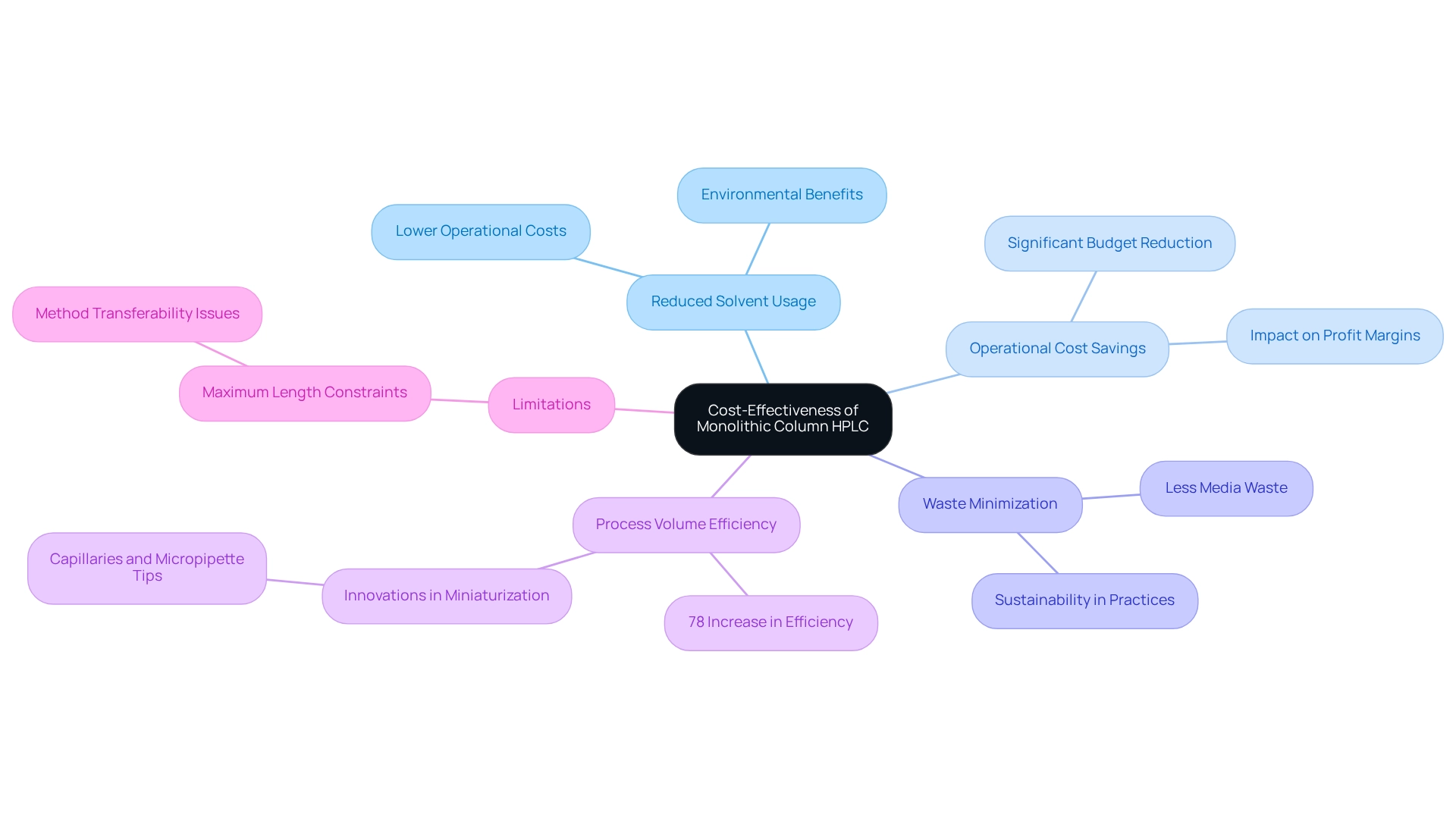
Monolithic column HPLC structures are highly regarded for their low backpressure and high permeability, which significantly reduces solvent usage during HPLC analyses. This reduction not only lowers operational costs but also minimizes waste generation, positioning these structures as environmentally friendly alternatives.
By employing large single structures, drug laboratories can achieve substantial reductions in solvent consumption, which often constitutes a significant portion of their operational budget. Indeed, studies reveal that the implementation of solid columns can yield an impressive 78% increase in process volume efficiency while generating considerably less media waste. Such advancements translate into notable cost savings for pharmaceutical companies, especially in an industry characterized by constrained profit margins.
Moreover, the , exemplified by the use of capillaries and micropipette tips for molecularly imprinted (MIP) monoliths, highlights ongoing innovations in this field, enhancing both efficiency and sustainability in laboratory practices. Furthermore, single-block materials are being utilized in solid-phase extraction (SPE) with commendable extraction efficiency, showcasing their versatility beyond HPLC applications.
However, it is crucial to acknowledge certain limitations, such as the maximum length of sections, which can influence method transferability. Despite these challenges, the integration of monolithic column HPLC with molecular imprinting technology represents a significant advancement in analytical capabilities, further reinforcing their role in contemporary drug laboratories.
Improved Resolution: Superior Separation of Compounds
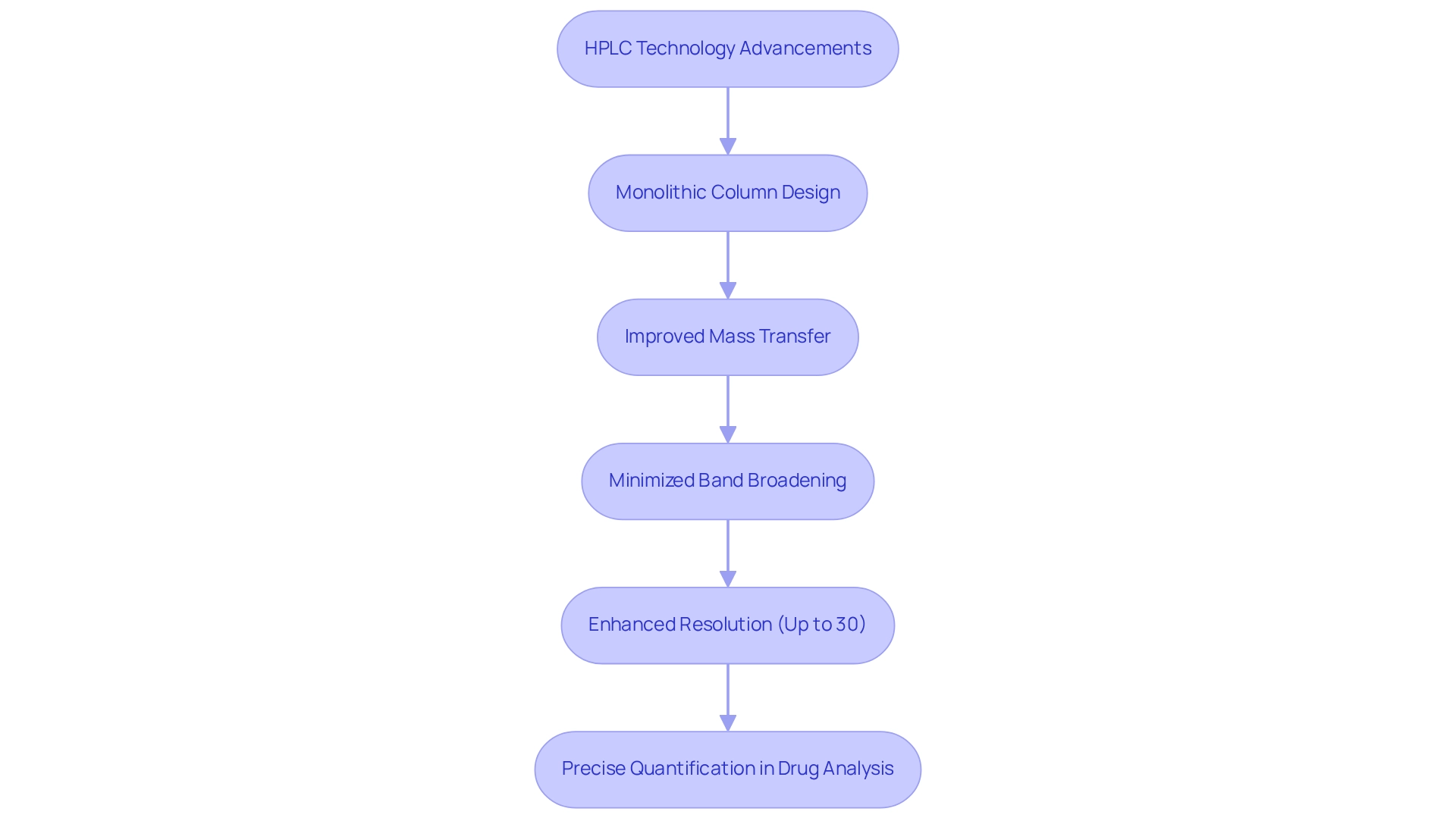
Monolithic column HPLC significantly enhances separation efficiency due to its innovative structure, which facilitates superior mass transfer and minimizes band broadening. This design leads to improved resolution of closely eluting compounds, a critical factor in pharmaceutical applications where precise identification and quantification of active ingredients are paramount. Improved resolution not only assists laboratories in meeting regulatory standards but also strengthens the reliability of test outcomes, thereby reinforcing the integrity of drug development processes.
Recent advancements in sample preparation techniques, based on sorption processes, have further highlighted the effectiveness of solid materials. These materials exhibit tunable properties and high permeability, contributing to more efficient analytical processes. In fact, studies indicate that the use of single-block supports can lead to resolution enhancements of up to 30% compared to conventional packed supports, underscoring their significance in pharmaceutical analysis. As the industry continues to evolve, the integration of such innovative technologies will be crucial for enhancing the efficiency and accuracy of compound analysis.
Additionally, it is important to consider that approximately 15% of paracetamol in blood serum is bound to plasma proteins, emphasizing the need for precise resolution in quantifying active ingredients in complex matrices. As mentioned by Nobuo Tanaka, 'We thank Karin Cabrera (Darmstadt, Germany) and Nobuo Tanaka (Kyoto, Japan) for the generous gift of the research sample solid structures,' highlighting the collaborative efforts in advancing HPLC technology. Moreover, while the monolithic column HPLC structures provide substantial benefits, it is crucial to recognize their limitations in protein analysis, maintaining a balanced viewpoint on their use. Overall, the significance of separation efficiency in drug analysis cannot be overstated, as it directly affects the accuracy and reliability of testing results.
Versatility: Adaptable for Diverse Pharmaceutical Applications
stands out for its remarkable versatility, serving a broad spectrum of applications in medical analysis, including the separation of small molecules, peptides, and proteins. Their capacity to accommodate various sample types without necessitating them as an optimal choice for laboratories seeking flexibility in analytical capabilities. This inherent adaptability empowers pharmaceutical labs to transition seamlessly between different analyses, ultimately maximizing their operational efficiency. By leveraging monolithic column HPLC, laboratories can enhance their analytical workflows, ensuring they remain at the forefront of scientific innovation.
User-Friendly: Simplified Operation and Maintenance
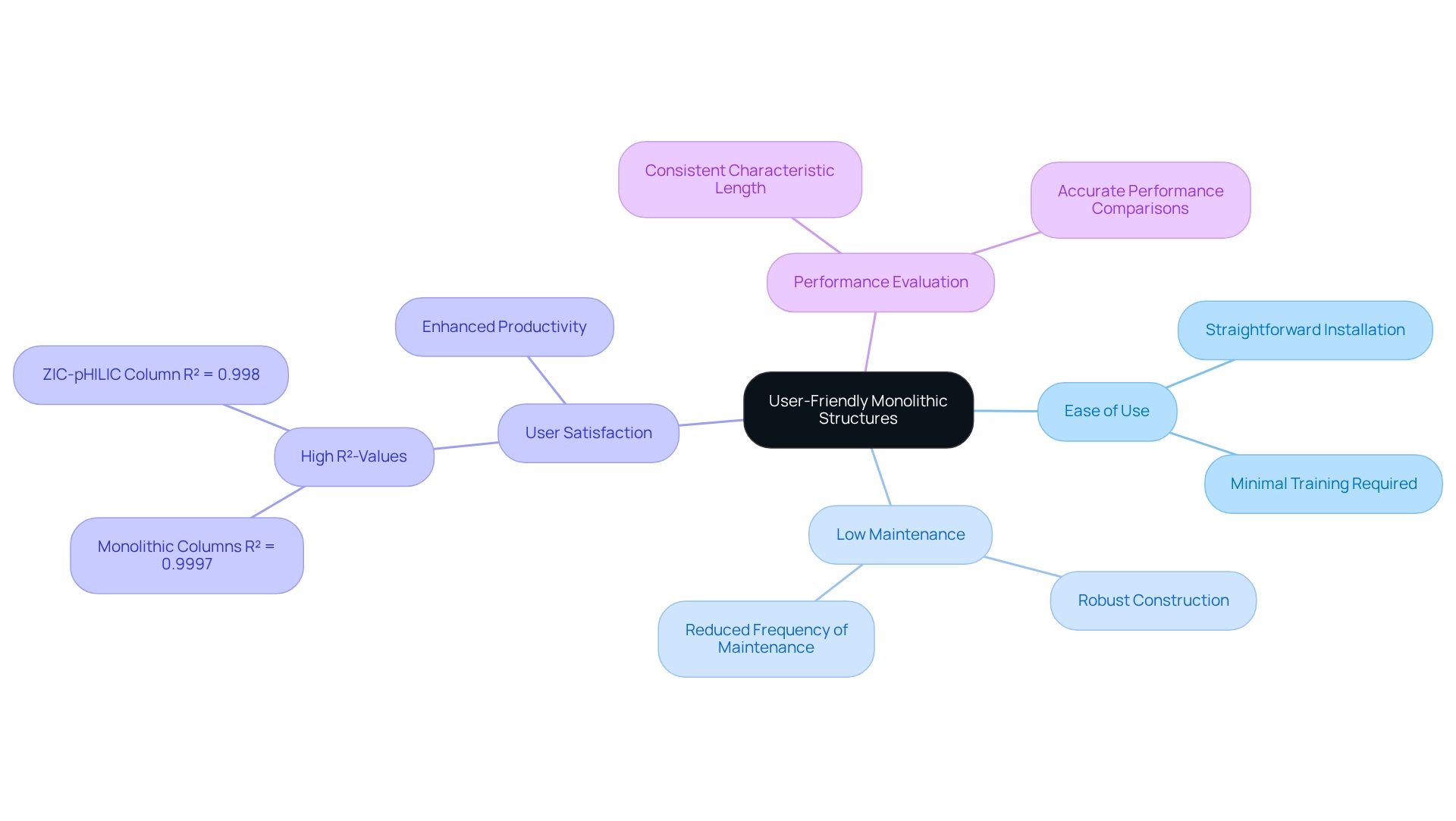
Monolithic structures are engineered for optimal ease of use, featuring straightforward installation and maintenance procedures that significantly enhance laboratory operations. Their robust construction minimizes the risk of damage and reduces the frequency of maintenance interventions, enabling laboratories to function efficiently.
User satisfaction statistics indicate that these sections achieve remarkable R²-values, such as 0.9997, underscoring their reliability in assessment tasks. This user-friendly design empowers laboratory staff to concentrate on evaluation tasks rather than troubleshooting equipment issues. As a result, labs can maintain high productivity levels, allowing staff to operate the equipment with minimal training.
Case studies reveal that the consistent characteristic length of solid structures is crucial for accurate performance evaluations, further underscoring their value in medical environments.
In summary, the operational simplicity and low maintenance associated with it as an exceptional choice for laboratories aiming to enhance efficiency and effectiveness in their testing processes.
Scalability: Easily Expandable for Growing Lab Needs
Monolithic structures are engineered for exceptional scalability, empowering laboratories to effortlessly enhance their analytical capacities as operational requirements evolve. These structures facilitate an increase in sample throughput and support larger-scale analyses without necessitating a complete system overhaul. Such adaptability is vital, especially in a landscape where the demand for efficient testing solutions is surging, propelled by a 20% increase in that challenge the recruitment of technical talent within the pharmaceutical sector. As the industry grapples with a shortage of qualified experts, the urgency for effective and reliable evaluation techniques intensifies.
For instance, Oakwood Labs, a leader in sustained-release medication development, has adeptly leveraged unified structures to refine their assessment processes. By utilizing these advanced frameworks, they have significantly improved their testing analysis capabilities, which are critical for their innovative Chroniject™ technology that delivers small molecules and peptides over extended durations. This scenario exemplifies how unified structures can bolster expanding analytical capabilities, enabling laboratories to efficiently meet the growing demands of drug research while adhering to stringent regulatory requirements.
As laboratories continue to expand, the flexibility of unified structures becomes increasingly essential. They not only accommodate the rising volume of samples but also enhance the precision and efficiency of analyses, aligning with the industry's focus on quality and efficiency. The AI in clinical trials market, projected to reach $4.9 billion by 2028, underscores the growing emphasis on innovative testing solutions that ensure quality and safety in drug development. Consequently, the use of monolithic column HPLC structures emerges as an invaluable asset for laboratories striving to maintain a competitive edge in the rapidly evolving pharmaceutical landscape.
Durability: Long-Lasting Performance in Rigorous Conditions
Monolithic structures, such as , are engineered from high-quality, robust materials specifically designed to withstand the demanding conditions of high-performance liquid chromatography. JM Science Inc. offers a diverse range of top-tier HPLC products, including Shodex and CapcellPak, ensuring exceptional durability that guarantees reliable performance over extended periods, even in challenging environments.
This remarkable resilience significantly reduces the frequency of replacements and repairs, thereby minimizing operational disruptions and associated costs. For drug laboratories, such reliability is essential for maintaining the integrity of analytical processes and ensuring compliance with stringent industry standards.
Additionally, the maintenance expenses associated with the monolithic column HPLC are often diminished, rendering them a cost-effective choice for laboratories focused on long-term performance and dependability. JM Science Inc. also supplies an array of HPLC fittings, titrators, and other accessories, further catering to the needs of drug research laboratories.
As Haibin Li notes, advancements in vertical structure technology are supported by substantial research and development efforts, enhancing their application in medical environments.
Regulatory Compliance: Meeting Industry Standards with Confidence
Monolithic pillars are meticulously engineered to meet the stringent regulatory standards of the drug industry. Their exceptional efficiency and reproducibility are pivotal in delivering reliable analytical results, crucial for compliance with regulatory frameworks established by the FDA and EMA. By utilizing robust structures, drug laboratories can confidently conduct their analyses, fully aware that they adhere to required protocols, thereby ensuring the safety and efficacy of their products.
As Gurudev Mallesha, ISO Lead Auditor, asserts, "Compliance becomes manageable when companies start early." This statement underscores the importance of from the outset, a fundamental aspect for maintaining regulatory standards and enhancing operational efficiency in drug analysis.
Overall Impact: Boosting Productivity and Efficiency in Pharmaceutical Labs
The incorporation of solid structures into pharmaceutical labs significantly enhances productivity and efficiency. Their advantages—ranging from faster analysis times and reduced solvent usage to improved resolution and user-friendliness—collectively enhance the overall analytical workflow. offers a diverse range of premium HPLC products, including Shodex and CapcellPak, along with titrators and Karl Fischer reagents, all designed to amplify these benefits.
As Patricia Regal notes in her research on recent advancements and applications of integrated structures, these technologies are crucial for achieving high performance across various applications. By integrating these advanced technologies, pharmaceutical labs can not only address their current challenges but also strategically position themselves for future growth and innovation in drug development and quality assurance.
Furthermore, the adaptability of single-piece structures is exemplified by their effective application in techniques such as SBSE, which successfully extracts penicillins from complex matrices like milk and honey. This adaptability highlights the potential of monolithic column HPLC, alongside JM Science's innovative medical devices, to enhance analytical capabilities across a broad spectrum of fields.
Conclusion
The advancements in high-performance liquid chromatography (HPLC) solutions, particularly through the adoption of monolithic columns, signify a pivotal advancement for pharmaceutical laboratories. By delivering faster analysis times, reduced solvent consumption, and superior resolution, these innovative tools empower labs to enhance productivity and efficiency. The integration of such technologies not only streamlines the analytical workflow but also ensures compliance with stringent regulatory standards, which is critical for maintaining the integrity of drug development processes.
Furthermore, the versatility and scalability of monolithic columns enable laboratories to adapt to evolving demands without compromising quality. As the pharmaceutical sector faces increasing pressure for timely and reliable testing, the durability and user-friendly design of these columns further bolster their appeal, allowing labs to concentrate on their core analytical responsibilities.
In summary, the shift towards advanced HPLC solutions, particularly those offered by JM Science Inc., is not merely a trend but a strategic necessity for pharmaceutical labs aiming to excel in a competitive landscape. By embracing these cutting-edge technologies, laboratories can optimize operations, enhance analytical capabilities, and ultimately contribute to the safety and efficacy of pharmaceutical products. The future of drug development and quality assurance relies on such innovations, underscoring the critical role that precision instruments play in the ongoing evolution of the pharmaceutical industry.




