Overview
This article delineates the various factors that significantly influence the elution order in chromatography, including:
- mobile phase composition
- temperature
- column chemistry
- flow rate
Each of these elements plays a pivotal role in determining the separation of compounds during analysis. For instance, discussions highlight how adjustments in solvent ratios, meticulous temperature control, and the implementation of gradient methods can optimize separation efficiency. Such refinements not only enhance the accuracy of chromatographic results but also underscore the importance of high-quality scientific instruments in laboratory settings. Ultimately, understanding these factors is crucial for achieving reliable and precise analytical outcomes.
Introduction
In the intricate world of chromatography, achieving optimal elution order is paramount for precise analytical outcomes. JM Science Inc. stands at the forefront of this field, offering a suite of high-performance liquid chromatography (HPLC) solutions designed to enhance separation efficiency and address the complex challenges faced by laboratories, particularly in pharmaceutical analysis.
As the landscape of chromatography evolves, understanding the multifaceted factors that influence elution order—ranging from mobile phase composition and temperature control to column chemistry and instrument calibration—becomes increasingly vital.
This article delves into these critical elements, exploring how advancements in technology and methodology can empower laboratory professionals to refine their chromatographic techniques and achieve reliable, reproducible results.
JM Science HPLC Solutions: Essential Tools for Optimizing Elution Order
JM Science Inc. offers a comprehensive array of high-performance liquid separation solutions that are crucial for optimizing separation sequences in analytical processes. Their specialized HPLC columns, particularly the esteemed Capcell Pak series, are meticulously engineered to enhance separation efficiency, addressing the intricate demands of pharmaceutical analyses.
Furthermore, JM Science supplies a range of titrators and Karl Fischer reagents, essential for achieving precise measurements within laboratory environments. By collaborating with industry leaders, JM Science ensures that laboratories benefit from cutting-edge technology and innovations in separation techniques, facilitating precise management of release conditions and the elution order.
As we approach 2025, optimizing the elution order and understanding retention mechanisms in reverse-phase HPLC has become increasingly vital for effective impurity assessment in pharmaceutical products. This trend highlights the imperative for laboratories to embrace that not only enhance analytical accuracy but also improve overall laboratory efficiency.
Additionally, the introduction of new Flom HPLC degassers further boosts the efficiency of HPLC systems, ensuring optimal performance. Real-world applications of JM Science's HPLC solutions demonstrate their effectiveness in improving separation outcomes.
For example, implementing effective maintenance practices for HPLC columns, such as thorough cleaning and proper storage in pure methanol or approximately 80% methanol-water solutions, has been shown to extend the lifespan of these instruments while sustaining their performance.
This dedication to quality and innovation solidifies JM Science's position as a leader in supplying essential tools for laboratories striving to achieve reliable and precise analytical results.
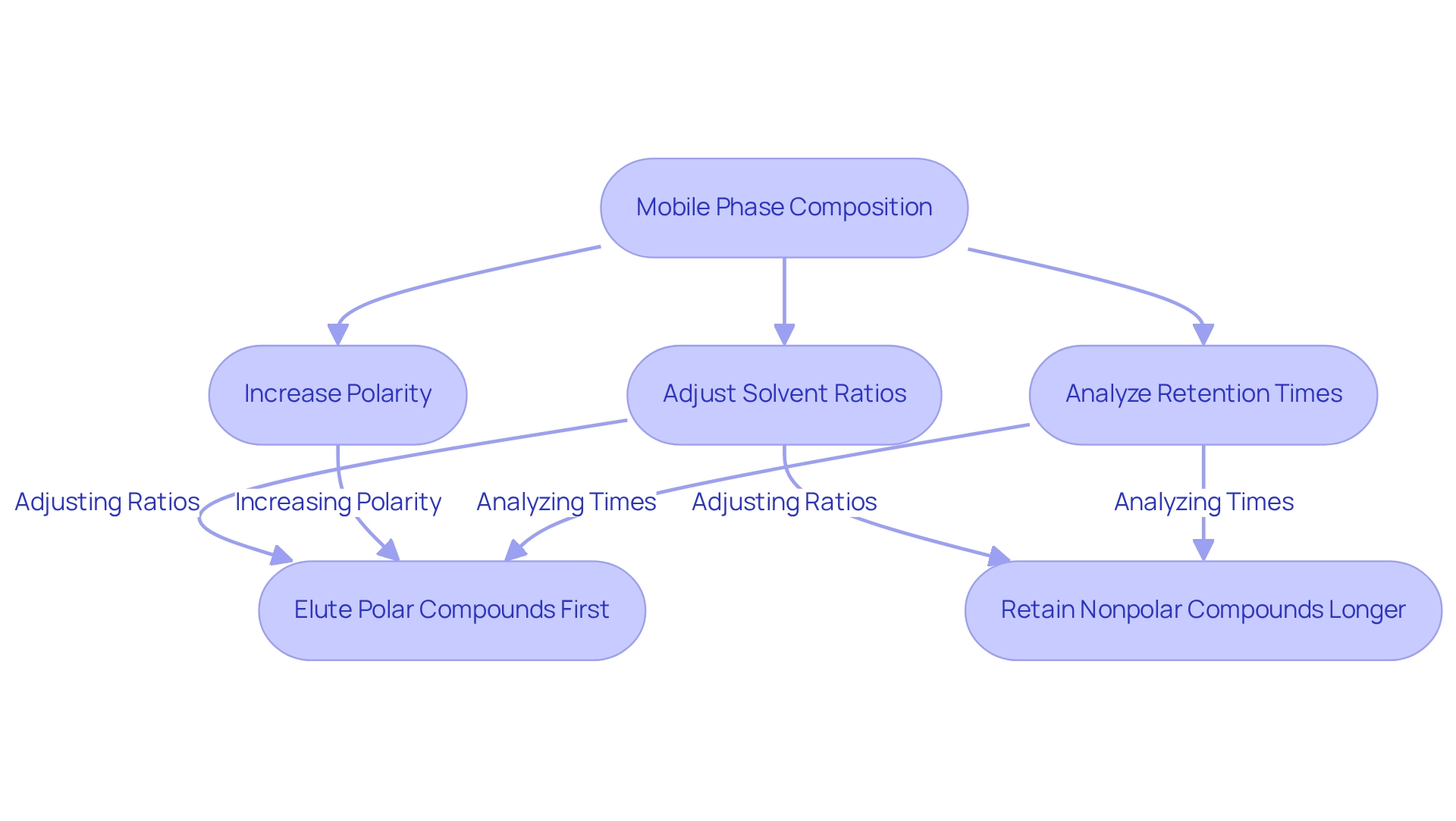
Mobile Phase Composition: Impact on Elution Order in Chromatography
The composition of the mobile component significantly affects the elution order in chromatography. By adjusting solvent ratios, particularly between water and organic solvents, analysts can effectively manipulate the polarity of the moving mixture, which subsequently impacts the retention times of various analytes. For example, typically elutes polar compounds first, while nonpolar compounds are retained longer.
This principle is vital for the creation and enhancement of methods, as evidenced by systematic techniques in that focus on refining variables such as mobile composition, gradient patterns, and flow rates. Dr. A. G. Jay asserts, "Understanding the nuances of interaction and affinity is not merely advantageous; it is essential for mastering the art of chromatography."
Anticipated advancements in stationary medium technology are expected to broaden the applicability of reverse phase chromatography (RPC), thereby improving its efficiency across various analytical contexts. Case studies indicate that iterative optimization of chromatographic factors leads to reliable and reproducible methods, underscoring the importance of comprehending mobile interactions for achieving optimal separation outcomes.
Adjustments in solvent ratios not only influence retention times but also play a critical role in establishing the elution order, making this understanding essential for laboratory professionals who aim to enhance their chromatographic methods. For practical application, laboratory managers should consider experimenting with specific solvent ratios tailored to the characteristics of their analytes to optimize their chromatographic techniques.
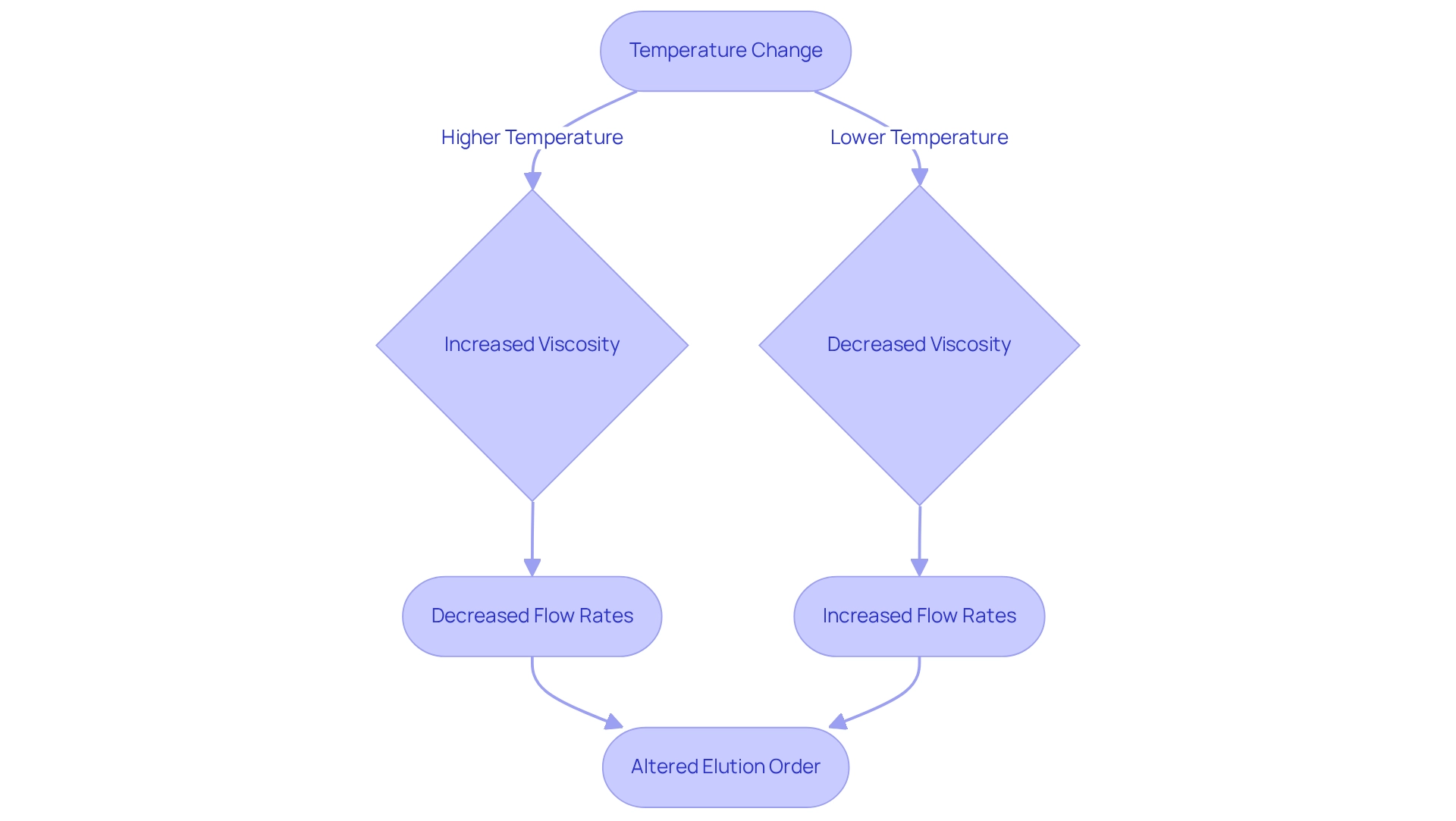
Temperature Control: How It Affects Elution Order in Chromatography
Temperature is a critical factor in separation techniques, significantly influencing the thickness of the moving liquid and the interactions between substances and the fixed medium. Elevated temperatures can reduce viscosity, leading to increased flow rates, which may alter the elution order during separation. Conversely, lower temperatures can enhance retention times for specific compounds. Thus, it is imperative to maintain a to ensure reproducibility in chromatographic analyses.
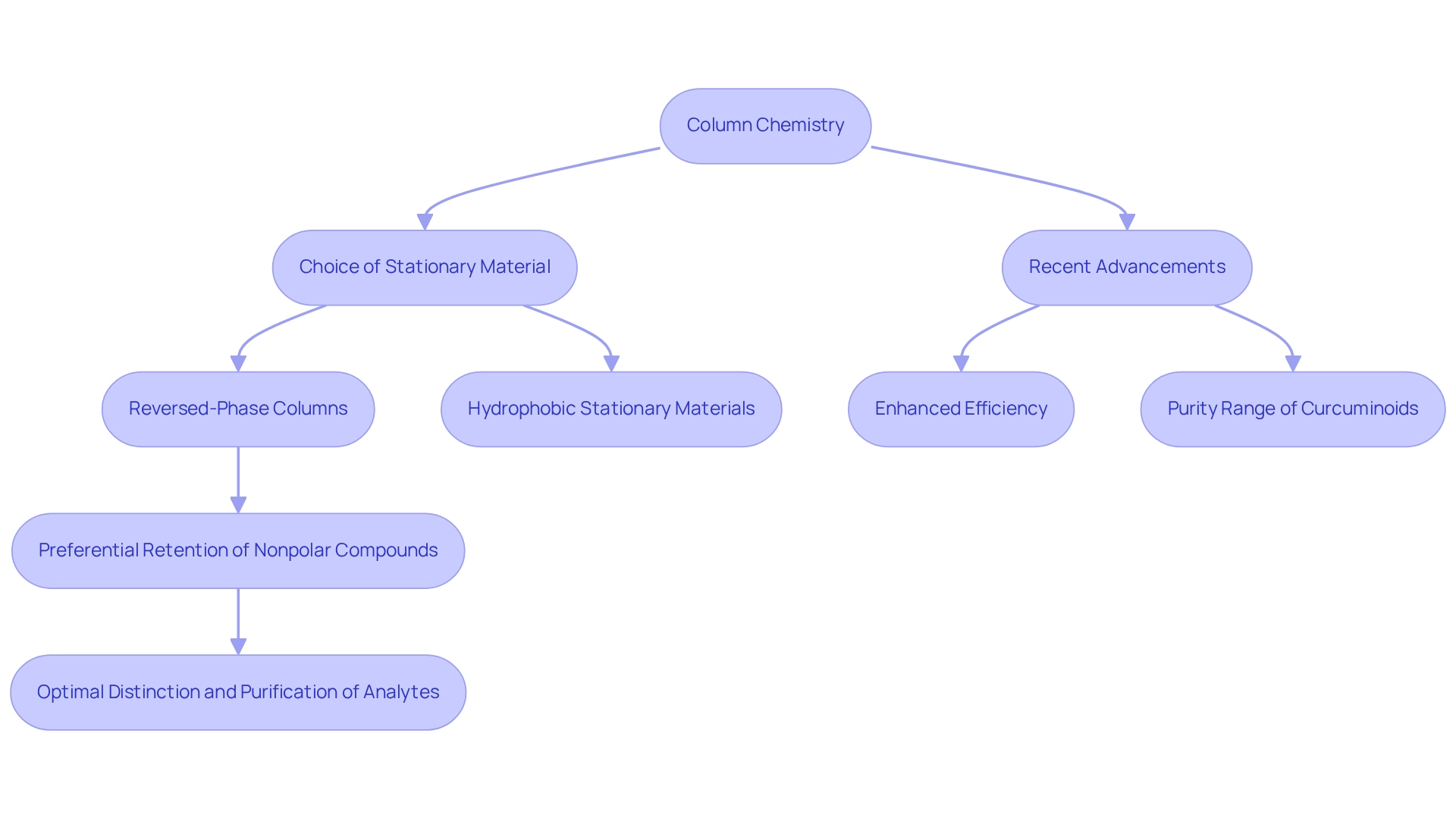
Column Chemistry: Its Role in Determining Elution Order
The chemistry of , especially the choice of stationary material, is crucial in determining the elution order of compounds. Reversed-phase columns, for instance, employ hydrophobic stationary materials that preferentially retain nonpolar compounds longer than their polar counterparts. This selective retention is critical for achieving optimal distinction and purification of analytes.
Recent advancements in column technology have further refined these interactions, with innovations in stationary substance materials significantly enhancing the efficiency of distinctions. The purity of isolated curcuminoids, for example, has been reported within the range of 92.4% to 95.45%, underscoring the effectiveness of modern chromatographic techniques.
Expert insights highlight that understanding the specific interactions between analytes and column chemistry is vital for selecting the most suitable column for a given application. This knowledge not only aids in optimizing the order of separation but also contributes to the overall success of chromatographic analyses. Furthermore, the ongoing development of stationary materials reflects contemporary trends in the field, emphasizing enhanced performance and reliability across diverse applications. Case studies illustrate the influence of column chemistry on the elution order, demonstrating how advancements in chromatographic theory, particularly through interdisciplinary collaboration, have resulted in practical guidelines that enhance the efficiency of mixture separations. Notably, the case study titled 'Advancements in Chromatographic Theory' exemplifies this collaboration, providing insights into how refined methodologies can enhance isolation processes.
As André M. Striegel, Scientific Advisor in the Chemical Sciences Division, notes, comprehending the domain of macromolecular isolation science is crucial for laboratory professionals. Staying informed about the latest developments in column chemistry is essential for laboratory managers striving to achieve precise and accurate results in their analyses.
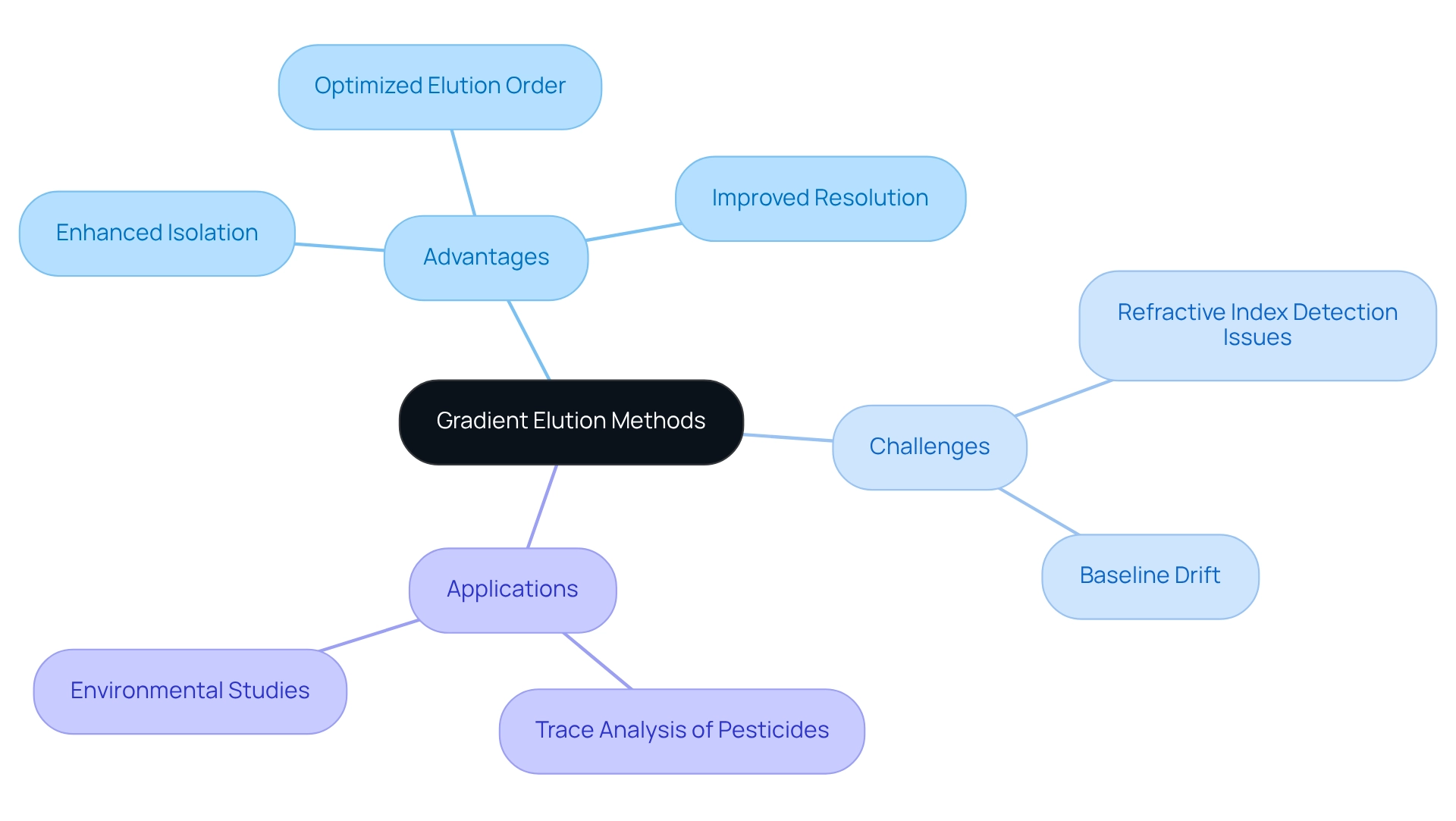
Gradient Elution Methods: Enhancing Separation and Elution Order
Gradient separation techniques are pivotal in analytical processes, as they entail the systematic variation of the mobile phase composition throughout the separation run. This dynamic approach enhances isolation and optimizes the order of solvent release by commencing with a weaker solvent and progressively increasing its potency. Such a strategy proves especially advantageous for complex mixtures, facilitating improved resolution and peak shape, which are essential for accurate analysis.
Recent advancements in have demonstrated enhanced control over gradient sharpness, further augmenting the efficacy of gradient separation. This precision is vital when isolating complex samples, such as those encountered in environmental studies, where understanding the elution order significantly improves the differentiation of closely related compounds. Real-world applications of gradient separation have yielded remarkable outcomes, particularly in the trace analysis of pesticides. The innovative 'spray-on' technique of sample application has been recognized for its substantial benefits in this domain. As noted by Bla¸dek Rostkowski, 'It was shown that the greatest advantages in the trace analysis of pesticides are attained through the use of the ‘spray-on’ technique of sample application,' underscoring the practical impact of gradient separation methods.
However, despite the numerous advantages gradient separation offers, it also introduces challenges. For example, the inability to utilize refractive index detection and potential baseline drift in chromatograms necessitates meticulous method development and validation to ensure reliable outcomes in high-performance liquid separation analyses. Addressing these challenges is crucial for laboratories aiming to harness the full potential of gradient separation techniques.
In summary, the integration of gradient techniques in analytical processes not only enhances efficiency but also aligns with contemporary trends in the field, making it an essential consideration for experts seeking to optimize their procedures.
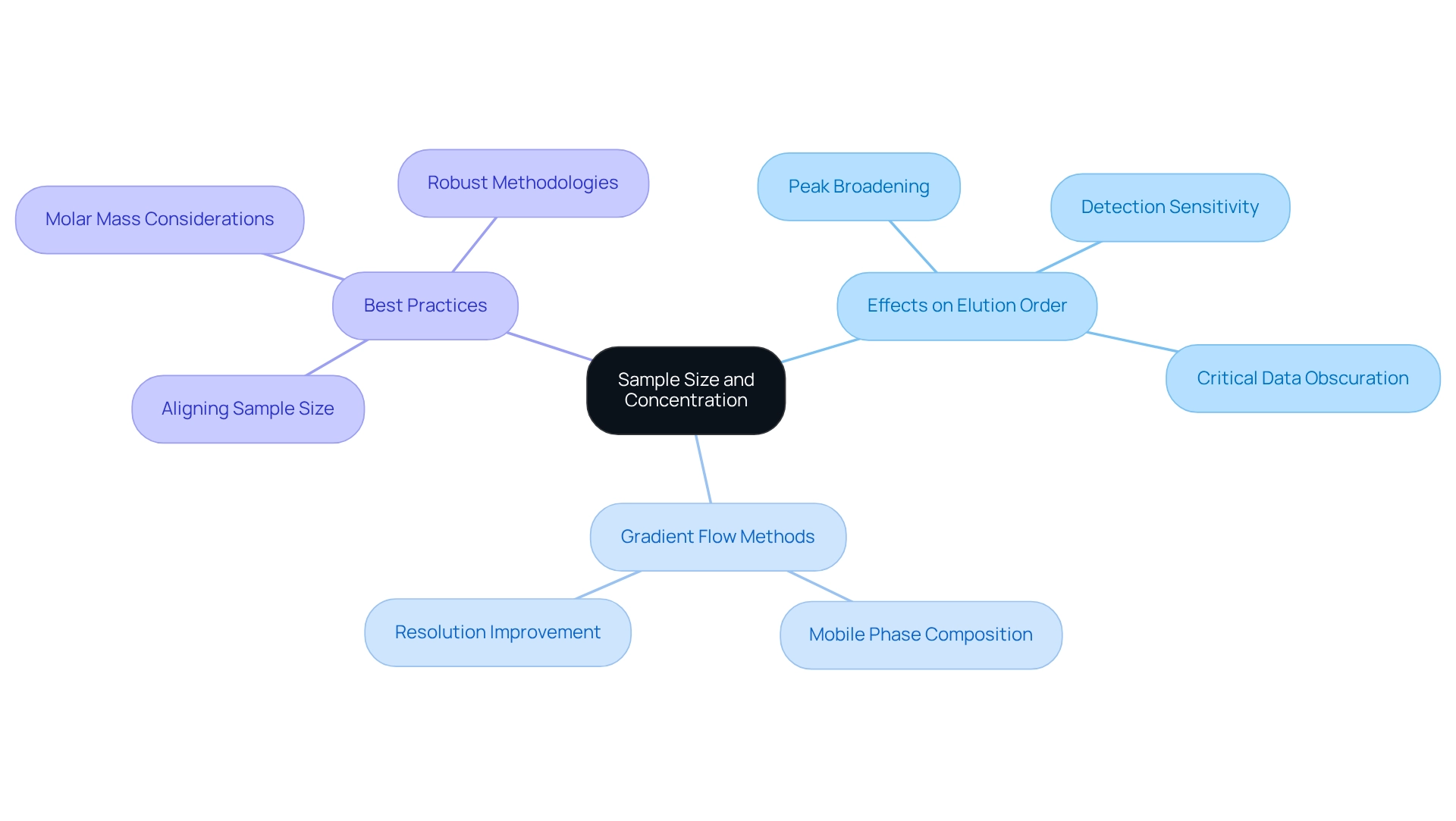
Sample Size and Concentration: Key Factors in Elution Order
The size and concentration of a sample are pivotal in establishing the elution order in this analytical technique. Elevated concentrations can lead to peak broadening and tailing, complicating result interpretation and potentially obscuring critical data. Conversely, excessively low concentrations may hinder detection sensitivity, making it challenging to identify analytes accurately. Therefore, optimizing both sample size and concentration is essential for achieving distinct and interpretable peaks that reflect the elution order in chromatographic analyses.
Research indicates that gradient flow methods can significantly enhance the resolution of complex mixtures by varying the mobile phase composition over time, which helps improve the elution order of the components. For instance, a recent validation study of for analyzing OZ in gel formulations demonstrated that careful control of sample concentration resulted in a reliable, rapid, and reproducible method suitable for routine quality control in laboratories.
Current best practices suggest that aligning sample size with the specific requirements of the analysis can maximize data quality while efficiently utilizing resources. As Daniela Held noted, "The higher the molar mass, the lower the concentration needs to be," which underscores the importance of understanding the relationship between sample characteristics and analytical outcomes. This approach not only improves the clarity of chromatographic results but also ensures that the method remains robust across varying sample conditions. Consequently, comprehending the impact of sample concentration on separation results is essential for laboratory managers seeking to enhance their analytical procedures.
pH Levels: Their Influence on Elution Order in Ion-Exchange Chromatography
In ion-exchange separation techniques, pH levels are fundamental in determining the charge of analytes, directly influencing their retention and release order. By adjusting the pH of the mobile phase, analysts can effectively manipulate the ionization state of compounds, optimizing their interaction with the stationary phase. Controlling the elution order is essential for achieving desired partitioning outcomes, as recent experimental studies have underscored the impact of pH on in mixed-mode techniques.
For instance, research conducted by Jannette Kreusser and colleagues at TU Kaiserslautern demonstrated that the steepest exponential decay of lysozyme loading occurred at pH 7.0, highlighting the critical role of pH in optimizing chromatographic processes. The findings suggest that various salts exhibit differing impacts on lysozyme loading due to their ionic characteristics, emphasizing the necessity for meticulous pH control.
Furthermore, the effect of pH on analyte charge is vital in ion-exchange separation. As pH levels fluctuate, the ionization of analytes changes, which affects the elution order and leads to significant variations in their retention times. This phenomenon is particularly pertinent in applications requiring high precision, such as pharmaceutical development and quality control.
Real-world examples illustrate the practical implications of pH manipulation. For instance, adjusting pH levels can enhance the separation of complex mixtures, facilitating more efficient purification processes. Recent studies, incorporating insights from Ting Yang, indicate that the application of pH-dependent descriptors in multiscale modeling can predict different modes of protein separation at any mobile-phase pH, potentially carrying substantial implications for downstream bioprocessing.
Moreover, the absence of systematic experimental studies on protein adsorption equilibria in mixed-mode separation methods highlights the continuous need for research in this domain, offering valuable insights that can be leveraged to enhance separation techniques and outcomes across various scientific disciplines.
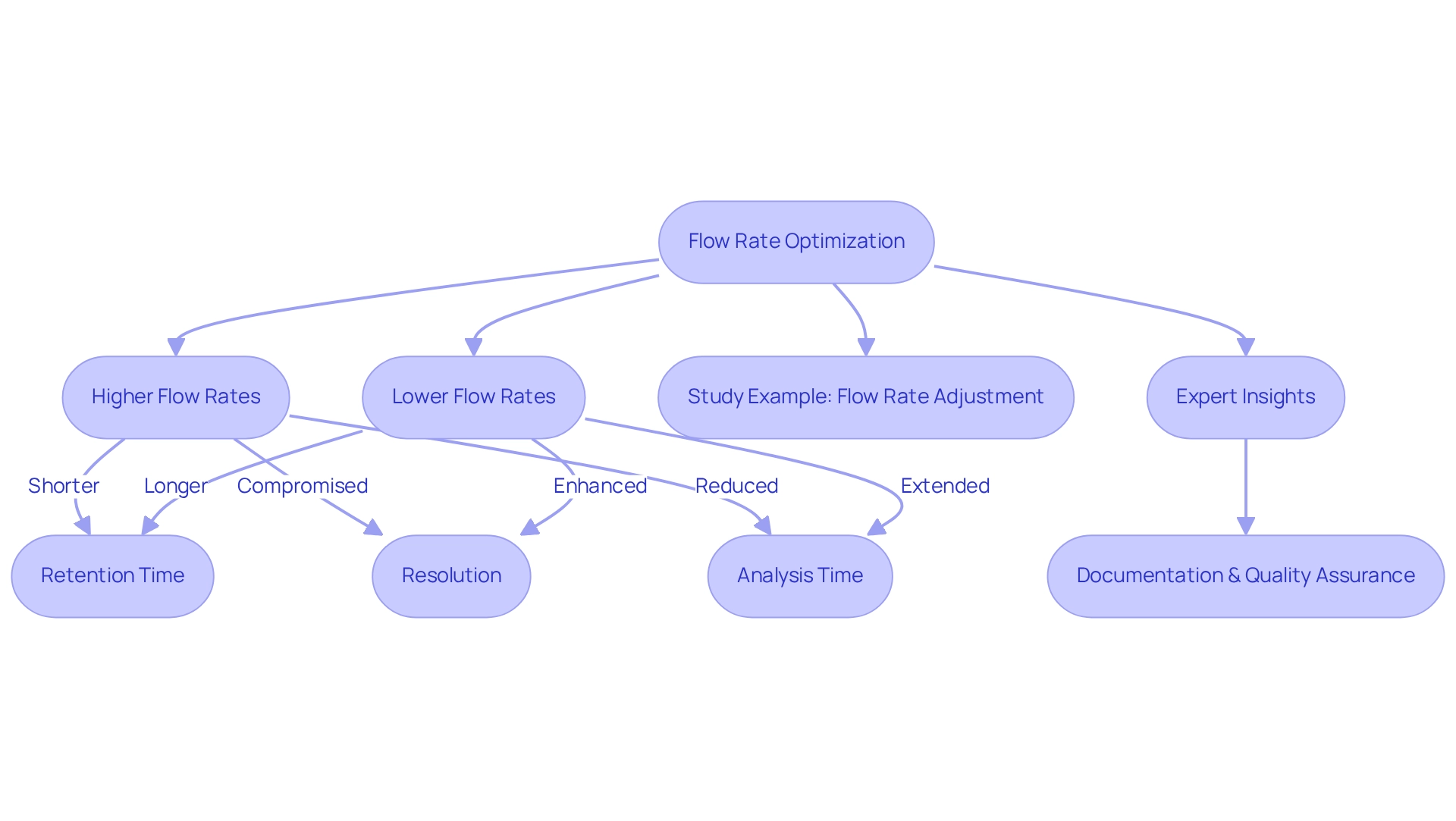
Flow Rate: Optimizing Conditions for Desired Elution Order
The flow rate of the mobile phase is a pivotal factor that influences the elution order in chromatography. Higher flow rates typically result in shorter retention times, expediting analysis but potentially compromising resolution. Conversely, reduced flow rates can enhance resolution, allowing for better distinction of compounds, albeit at the cost of extended analysis time. Striking the right balance between efficiency and quality of distinction is crucial during method development.
Optimizing flow rate transcends theoretical considerations; it has tangible implications for chromatographic performance. For instance, a study indicates that adjusting the flow rate from 0.7 to 0.8 mL/min necessitates a corresponding change in gradient time from 35 to 30.6 minutes to maintain system suitability. Such adjustments underscore the necessity of monitoring effects on quality of division to ensure optimal results.
Expert insights emphasize that if the underlying causes of division problems remain unrecognized or unresolved, flow-rate modifications may be essential, particularly in high-performance liquid analysis, where accuracy is paramount. As John W. Dolan, an editor of LC Troubleshooting, aptly notes, documenting these adjustments is vital for upholding quality assurance in laboratory practices.
Case studies, such as the one titled ," illustrate the complexities associated with adjusting flow rates and their effects on selectivity and peak spacing. Current best practices advocate for a systematic approach to flow rate optimization, ensuring that the elution order achieves desired separation sequences while maintaining high standards of analytical performance in laboratories.
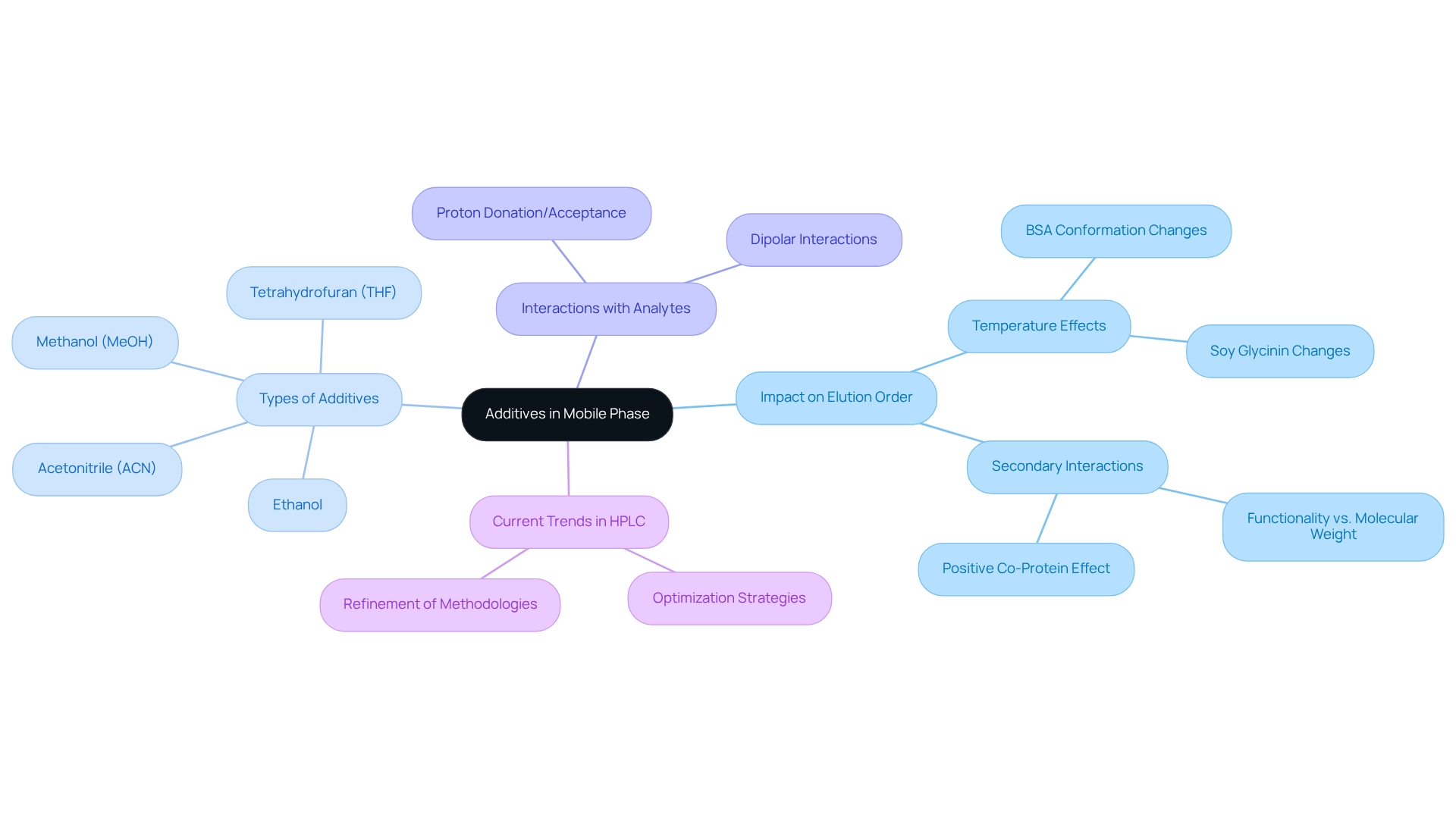
Additives in Mobile Phase: Modifying Elution Order in Chromatography
Additives in the mobile mixture are pivotal in modifying in chromatography, which significantly impacts the efficiency of analyte differentiation. The incorporation of salts or organic modifiers can enhance the solubility of specific compounds, resulting in improved retention and separation. For instance, research indicates that the conformation of proteins, such as bovine serum albumin (BSA), can vary under different conditions, particularly noting that the conformation of BSA and soy glycinin altered at lower temperatures in 50% (v/v) ethanol compared to water. This alteration affects their interaction with the stationary phase, ultimately influencing their separation sequence.
In practical applications, the utilization of modifier molecules in reversed-phase high-performance liquid chromatography (RP-HPLC) systems has revealed that various solvents, including methanol (MeOH), acetonitrile (ACN), and tetrahydrofuran (THF), demonstrate distinct capabilities in interacting with solutes. These interactions can either donate or accept protons, thereby affecting the retention times of analytes during analysis. This concept was highlighted in a study employing a solvent selectivity triangle based on normalized solvatochromic Kamlet–Taft parameters, illustrating how the choice of modifier can substantially alter the separation profile.
Furthermore, the effects of additives transcend simple solubility enhancements. Secondary interactions, influenced by the functionalities of analytes rather than just their molecular weight, can lead to unexpected changes in elution order. For example, the positive co-protein effect observed with certain proteins during heat treatment emphasizes the necessity of comprehending these interactions when optimizing chromatographic methods. He et al. underscored the influence of stationary substances and pore dimensions on the efficacy of size-based sorting, further highlighting the complexity of these interactions.
Current trends in HPLC optimization stress the strategic application of mobile phase additives to attain desired separation outcomes. By meticulously selecting and adjusting these additives, analysts can refine their methodologies, ensuring optimal performance and reproducibility in chromatographic analyses.
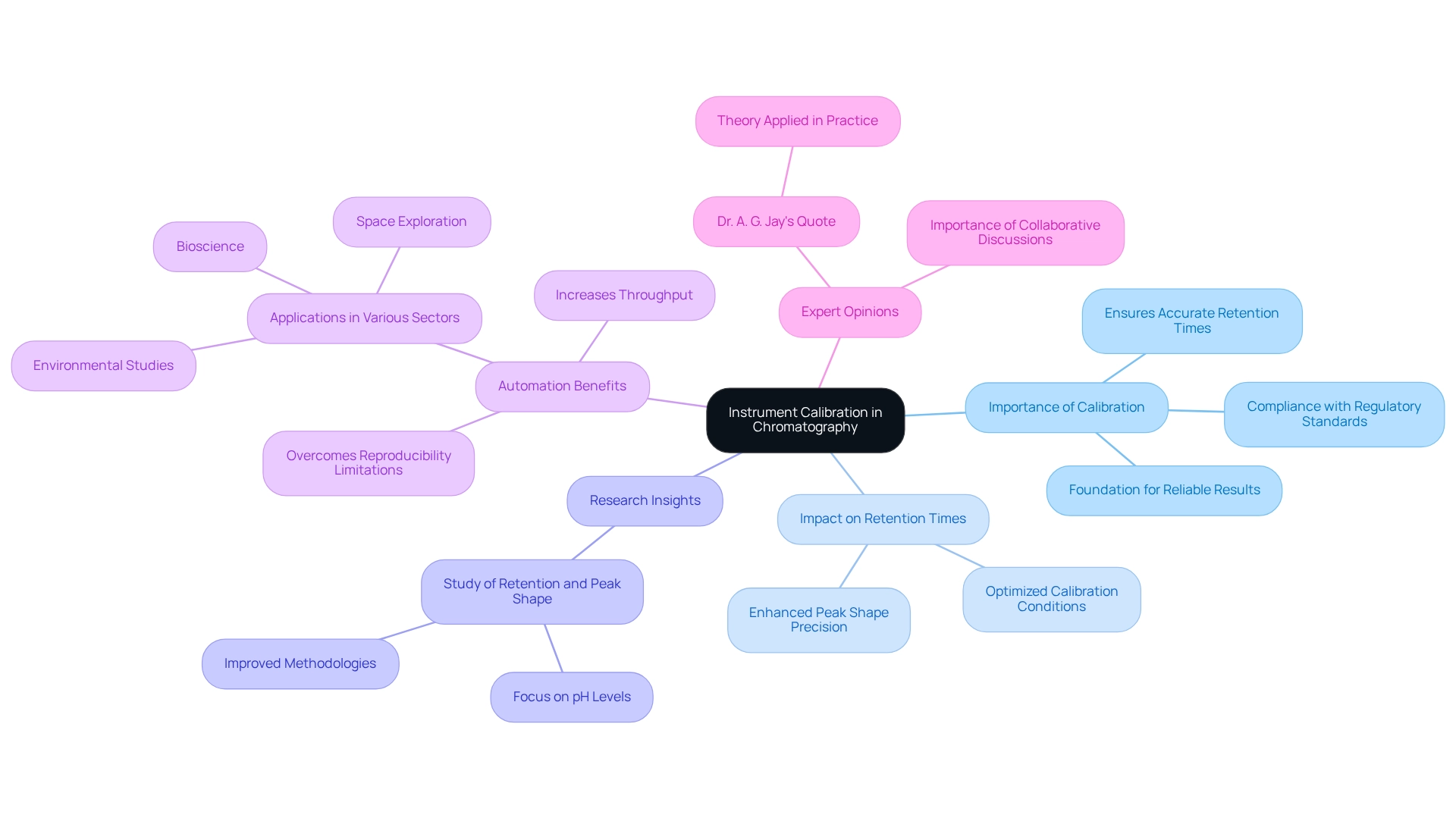
Instrument Calibration: Ensuring Accurate Elution Order in Chromatography
Instrument calibration is a crucial phase in ensuring precise separation sequences in analytical techniques. Regular calibration of HPLC systems is essential for maintaining the accuracy of retention time measurements, which is vital for reproducibility. Implementing a robust calibration protocol is imperative for laboratories aiming to achieve reliable results and comply with regulatory standards.
In 2025, the significance of HPLC calibration is highlighted by a study titled "Study of Retention and Peak Shape in Hydrophilic Interaction Chromatography," which explored retention behavior across various pH levels. This research revealed that optimized calibration conditions can significantly enhance peak shape and retention time precision, demonstrating how meticulous calibration practices lead to improved methodologies in separation science.
Furthermore, statistics indicate that automating analytical workflows, including mass spectrometry, addresses limitations related to reproducibility and throughput. This underscores the necessity for precise instrument calibration, as regular calibration not only enhances the reliability of results but also increases throughput, tackling common challenges faced by laboratories.
Expert insights further reinforce this perspective, with professionals affirming that effective calibration protocols are foundational for achieving consistent elution order. As Dr. A. G. Jay aptly stated, "Theory becomes profound when applied in practice; chromatography is no exception." This emphasizes the need to apply theoretical knowledge through rigorous calibration practices to ensure . Additionally, engaging in collaborative discussions and networking with peers can unveil valuable insights into chromatographic challenges, further highlighting the critical role of calibration in the field.
Conclusion
The intricate factors influencing elution order in chromatography are pivotal for achieving precise analytical results. JM Science Inc. offers a comprehensive range of high-performance liquid chromatography (HPLC) solutions designed to enhance separation efficiency, particularly in pharmaceutical analyses. Essential elements such as mobile phase composition, temperature control, column chemistry, and instrument calibration significantly impact the retention times of analytes, making them critical considerations for laboratory professionals.
Furthermore, a deep understanding of gradient elution methods, along with the effects of sample size, concentration, and pH levels, contributes to the optimization of chromatographic techniques. Technological innovations, including advanced column materials and improved flow rate control, facilitate superior separation and yield more reliable outcomes. Additionally, the strategic incorporation of additives in the mobile phase can modify elution orders, equipping analysts with further tools to refine their methodologies.
In conclusion, mastering the complexities of chromatography necessitates a comprehensive approach that integrates technological advancements with a thorough understanding of foundational principles. By leveraging these insights and employing cutting-edge HPLC solutions, laboratories can attain accurate, reproducible results that meet the evolving demands of analytical chemistry. The continuous development in this field promises to empower professionals to enhance their techniques and improve the reliability of their analytical processes.




