Overview
The article underscores the essential applications of chromatography within pharmaceutical laboratories, highlighting its pivotal role in drug development, quality control, and various analytical processes. High-performance liquid chromatography (HPLC) emerges as an indispensable tool for guaranteeing the precision and safety of pharmaceuticals. It enables the accurate separation and analysis of compounds, which is vital for adherence to regulatory standards and the integrity of drug formulations. By ensuring these critical functions, HPLC not only supports compliance but also enhances the overall reliability of pharmaceutical products.
Introduction
Chromatography serves as a cornerstone in the pharmaceutical industry, playing a pivotal role in ensuring the safety, efficacy, and quality of drugs and vaccines. As laboratories strive for precision in drug development and compliance with stringent regulations, the applications of chromatography are not only expanding but also evolving. However, with rapid advancements in technology and the increasing complexity of formulations, pharmaceutical labs face the challenge of effectively leveraging these techniques to meet future demands. This article explores ten essential chromatography applications that are shaping the landscape of pharmaceutical research and development, highlighting their significance in enhancing drug safety and efficacy.
JM Science HPLC Solutions: Precision in Chromatography Applications
JM Science offers a comprehensive array of high-performance liquid chromatography solutions, meticulously tailored to meet the stringent standards of pharmaceutical laboratories. These sophisticated instruments are vital for the , which is essential for ensuring accuracy in drug development and quality control processes. JM Science's high-performance liquid chromatography systems enhance sensitivity and reproducibility, establishing them as indispensable tools in analytical chemistry.
In addition to liquid chromatography solutions, JM Science provides a diverse selection of high-quality titrators, Karl Fischer reagents, and advanced medical devices, including electronic stethoscopes that facilitate remote patient monitoring. This technological integration supports pharmaceutical applications and aligns with the dynamic landscape of healthcare, where remote diagnostics are increasingly critical.
High-performance liquid chromatography is essential in drug development, especially in chromatography applications for the assay of active pharmaceutical ingredients and the identification of impurities. As the pharmaceutical sector demands high-quality medications, the precision offered by high-performance liquid chromatography becomes paramount. Industry leaders assert that precision in chromatography is vital for maintaining the integrity of drug formulations and ensuring compliance with regulatory standards.
Looking ahead to 2025, the chromatography applications of high-performance liquid chromatography in drug development are expected to expand, driven by the growing demand for personalized medicine and the increasing complexity of drug formulations. The ability of high-performance liquid chromatography to deliver reliable outcomes underscores its significance in the pharmaceutical industry, where the stakes are high and the margin for error is minimal. As market dynamics evolve, JM Science remains committed to providing state-of-the-art chromatography applications and high-quality scientific instruments that meet the changing demands of pharmaceutical laboratories, solidifying its position as a trusted partner in enhancing drug development precision.
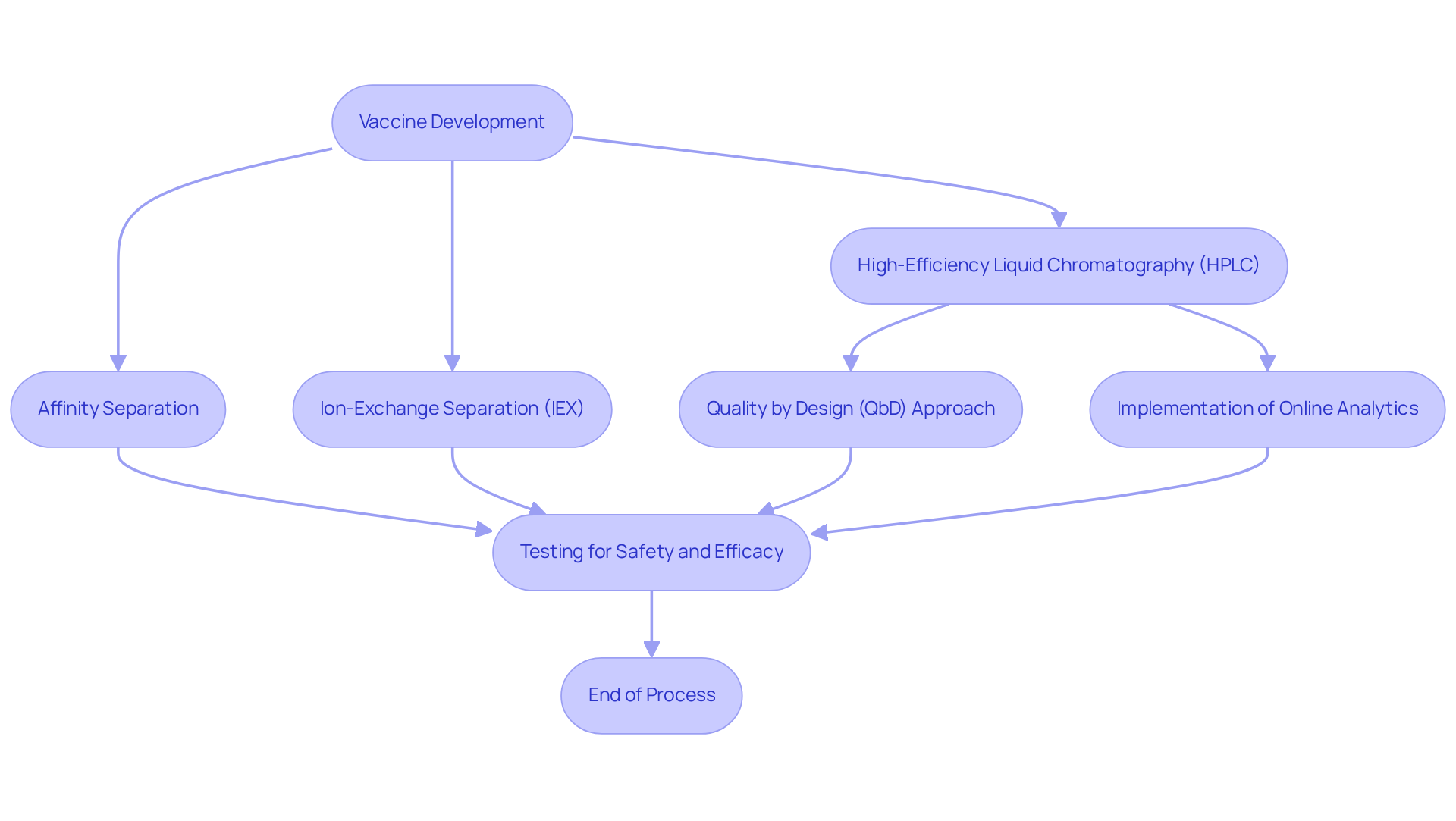
Vaccine Development: Utilizing Chromatography for Enhanced Safety and Efficacy
Chromatography is pivotal in the development of immunizations, facilitating the separation and analysis of complex biological mixtures. Techniques such as high-efficiency liquid chromatography (HPLC) are employed to refine immunization components and assess their strength and purity. Approximately 90% of immunizations utilize separation techniques during their development, underscoring their critical role in meeting stringent safety and effectiveness standards prior to public endorsement. This thorough testing process not only guarantees that immunizations are safe for administration but also enhances their efficacy in combating diseases.
Current best practices in employing HPLC for immunization safety and efficacy testing include the implementation of a quality by design (QbD) approach. This methodology has been adopted to develop rapid and sensitive for quality control, exemplified by those used for the 4CMenB immunization. By ensuring that processes are designed to meet predefined objectives, this approach significantly improves the reliability of quality control results for immunizations.
Moreover, advancements in separation methods, including affinity separation and ion-exchange separation (IEX), enable the rapid creation and production of substantial quantities of immunizations, ensuring they are free from impurities. The integration of online and inline analytics, alongside the increasing importance of Raman spectroscopy, is expected to further streamline processes, reducing analysis duration and enhancing production cycle times.
The rapid development of the Pfizer-BioNTech and Oxford-AstraZeneca vaccines within just 12 months following the discovery of the SARS-CoV-2 virus exemplifies the essential role of separation techniques in urgent immunization development scenarios. As the landscape of immunization advancement evolves, the chromatography applications of HPLC and other chromatographic methods are anticipated to expand, reinforcing their importance in producing high-quality, effective immunizations that bolster public health initiatives. Pharmaceutical lab supervisors are encouraged to explore these innovative separation techniques to enhance their vaccine development processes.
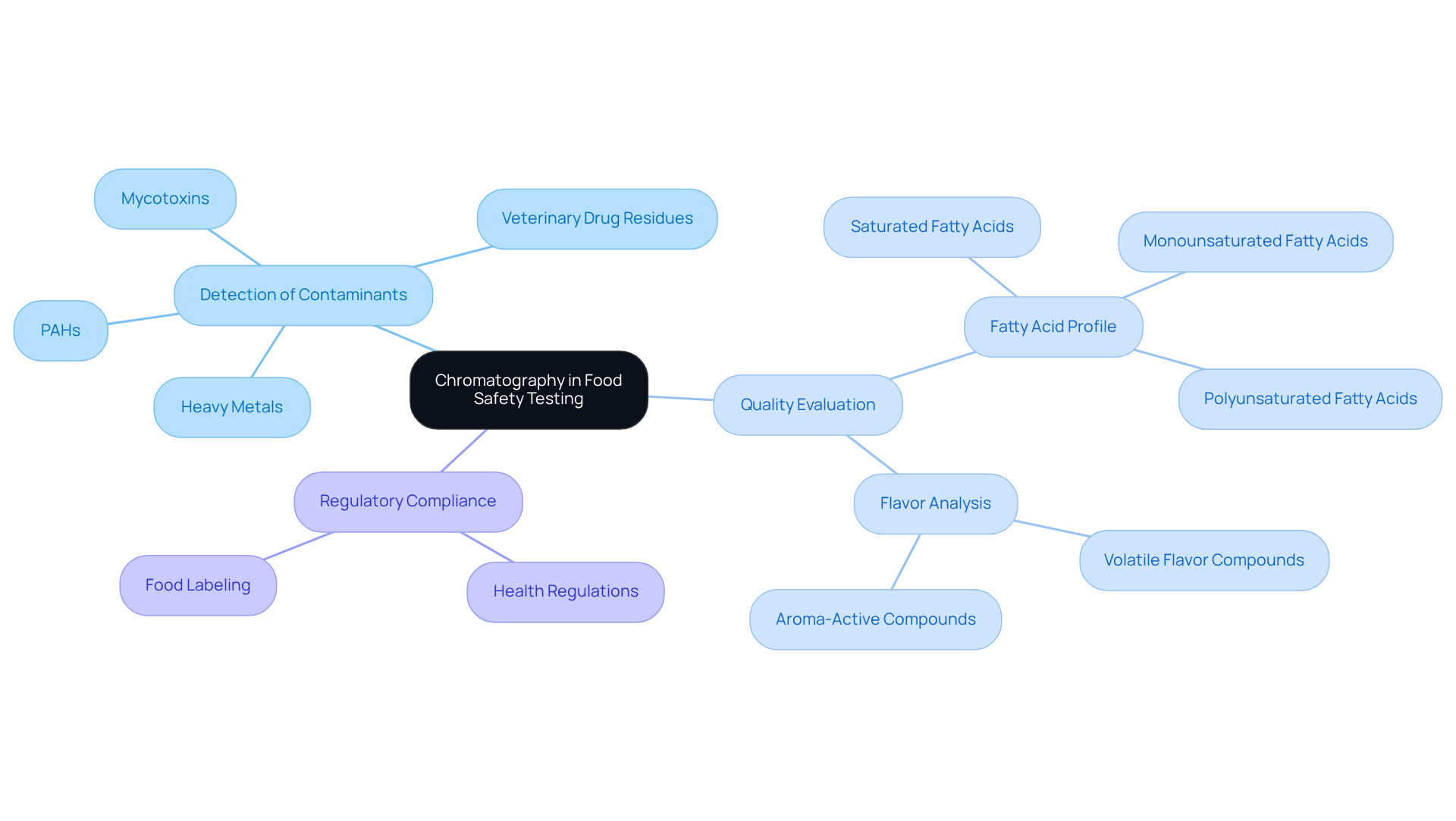
Food Safety Testing: Chromatography's Role in Ensuring Quality and Compliance
In the realm of food safety testing, chromatography applications are pivotal in detecting contaminants and ensuring compliance with health regulations. Methods such as gas separation and liquid separation are employed to scrutinize food products for pesticide residues, additives, and other harmful substances. This application not only safeguards consumer health but also aids manufacturers in adhering to regulatory standards. Furthermore, gas separation techniques provide crucial insights for food labeling and nutritional evaluation, which are vital for fostering transparency and consumer confidence.
Gas chromatography (GC) is instrumental in evaluating the quality and authenticity of oils by analyzing specific fatty acid markers, thereby determining the fatty acid profile, including saturated, monounsaturated, and polyunsaturated fatty acids. Additionally, GC techniques like solid-phase microextraction (SPME) and dynamic headspace analysis enhance the flavor analysis process by characterizing volatile flavor compounds and fragrances in food and beverage products, ensuring that these products meet quality expectations.
The chromatography applications of separation techniques extend to identifying various contaminants in food, including mycotoxins, veterinary drug residues, polycyclic aromatic hydrocarbons (PAHs), and heavy metals. By providing valuable insights into food quality and safety, these analytical methods not only protect public health but also assist manufacturers in meeting stringent food safety compliance standards. This comprehensive approach to food examination underscores the essential role of in maintaining high standards within the food industry.
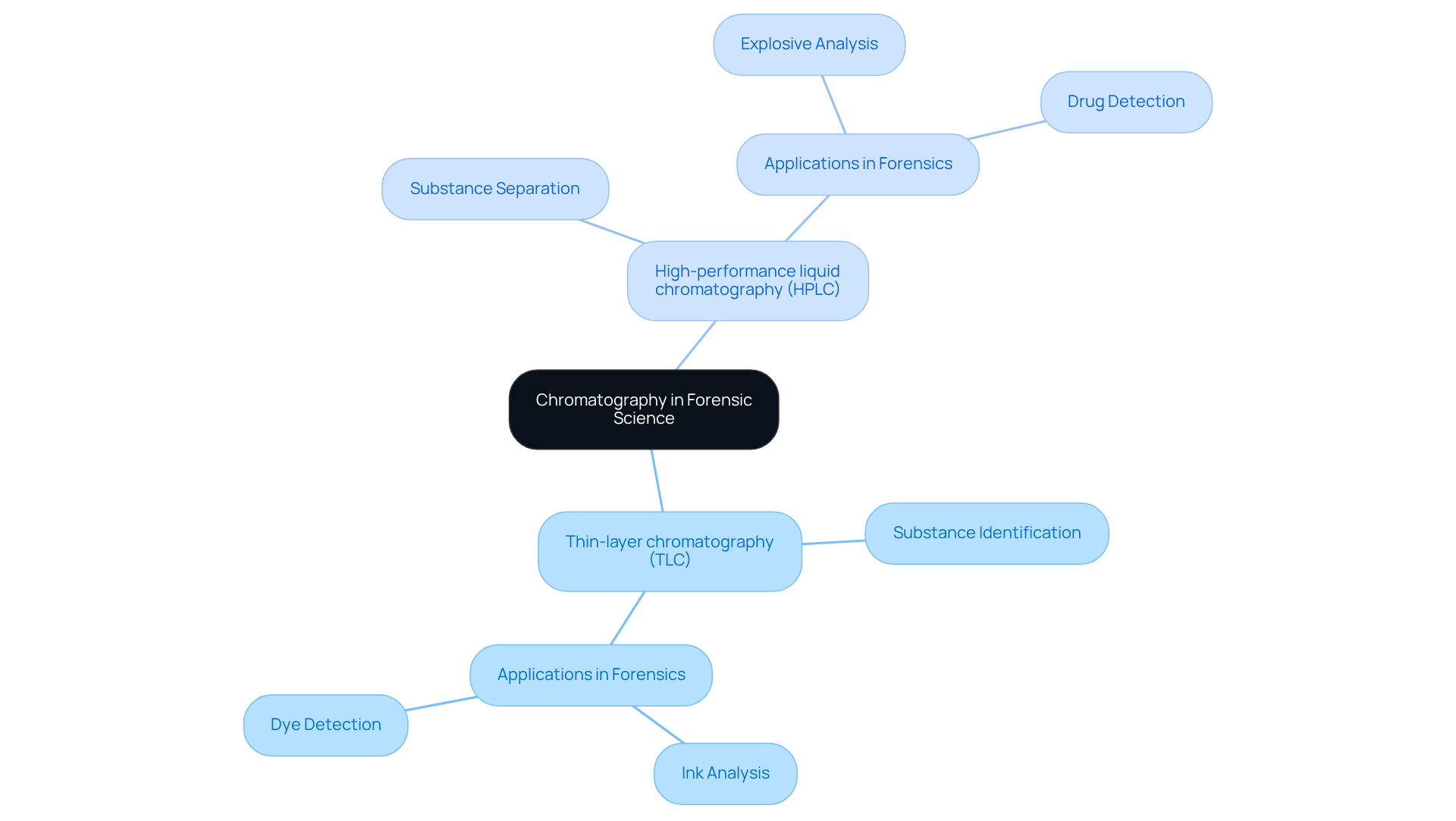
Forensic Science: Leveraging Chromatography for Substance Analysis
Chromatography applications stand as a cornerstone technique in forensic science, crucial for the meticulous analysis of substances encountered at crime scenes. By effectively separating complex mixtures, chromatography applications allow forensic scientists to accurately identify drugs, toxins, and other chemical compounds. Frequently employed methods, such as:
are among the key chromatography applications that provide reliable evidence in legal matters. This underscores the vital role of , reinforcing the need for high-quality scientific instruments in laboratory settings.
Pharmaceutical Research: Chromatography in Drug Formulation and Quality Control
In pharmaceutical research, separation techniques are essential to drug formulation and quality control processes. They enable researchers to of drug formulations, ensuring that active ingredients are present in the correct concentrations. A prime example is the AQ-300 Coulometric Karl Fischer Titrator, offered by JM Science Inc., which plays a crucial role in drug and medicine testing, particularly in compliance with the Japanese Pharmacopoeia.
Furthermore, chromatography applications are instrumental in monitoring the stability of pharmaceutical products over time, thereby maintaining compliance with regulatory standards and ensuring patient safety. JM Science Inc. also provides a comprehensive range of premium scientific instruments, including HPLC solutions and innovative medical devices, further supporting the pharmaceutical industry in achieving high-quality standards.

Environmental Analysis: Monitoring Pollutants with Chromatography Techniques
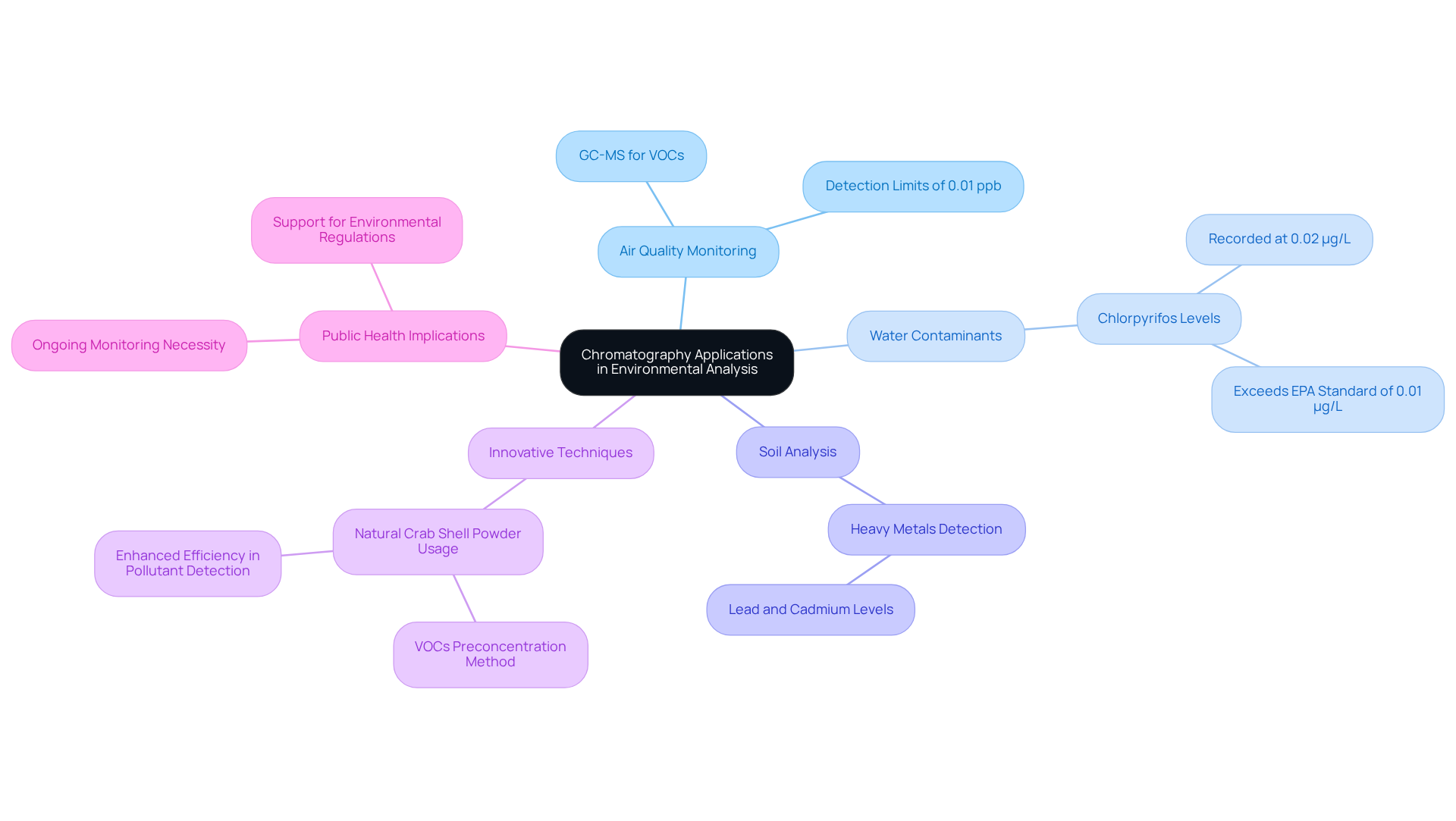
Chromatography applications are essential for environmental evaluation, especially in detecting contaminants in air, water, and soil. Laboratories can bolster environmental protection initiatives by utilizing chromatography applications like gas chromatography-mass spectrometry (GC-MS) to identify trace levels of hazardous substances. Recent advancements have led to the creation of fully that can detect volatile organic compounds (VOCs) at limits as low as 0.01 ppb, highlighting the precision of these methodologies in air quality monitoring.
Furthermore, research indicates that water samples can show elevated pesticide levels, with chlorpyrifos recorded at 0.02 µg/L, surpassing the EPA standard of 0.01 µg/L. This underscores the critical necessity for ongoing monitoring to protect public health.
In practical chromatography applications, innovative techniques, including the use of natural crab shell powder for VOC preconcentration, have demonstrated effectiveness in analyzing ambient air samples and showcasing enhanced efficiency in pollutant detection.
Overall, chromatography applications not only facilitate compliance with environmental regulations but also significantly support public health initiatives aimed at reducing pollution.
Clinical Diagnostics: Chromatography's Impact on Biomarker Analysis
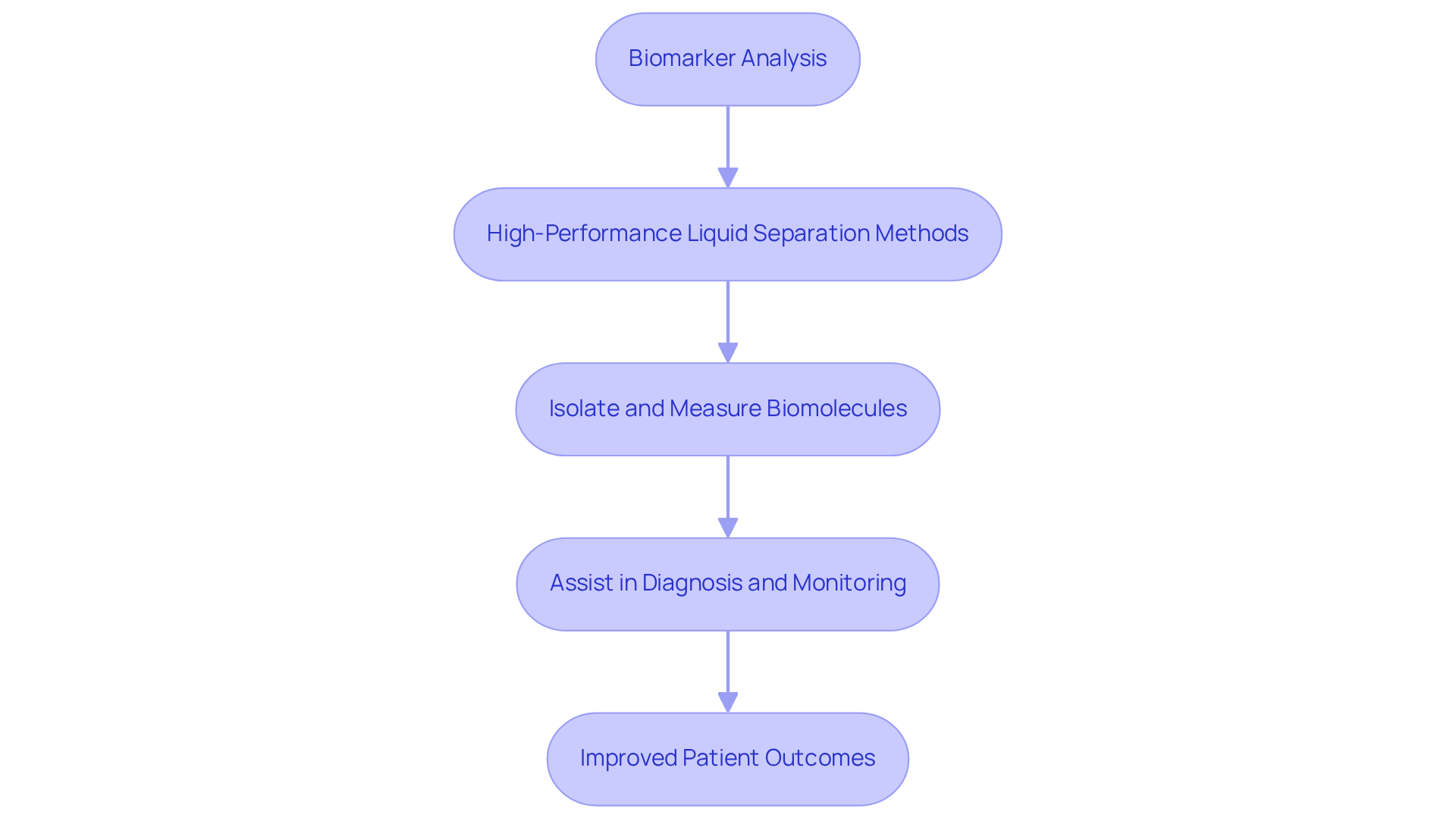
In clinical diagnostics, separation techniques play a crucial role in analyzing biomarkers that indicate disease states. High-performance liquid separation methods are employed to effectively isolate and measure biomolecules, thereby assisting in the diagnosis and monitoring of various health issues. This application significantly enhances the , ultimately leading to improved patient outcomes. By leveraging these advanced techniques, healthcare professionals can make more informed decisions, ensuring that patients receive the best possible care.
Beverage Testing: Ensuring Quality and Safety through Chromatography
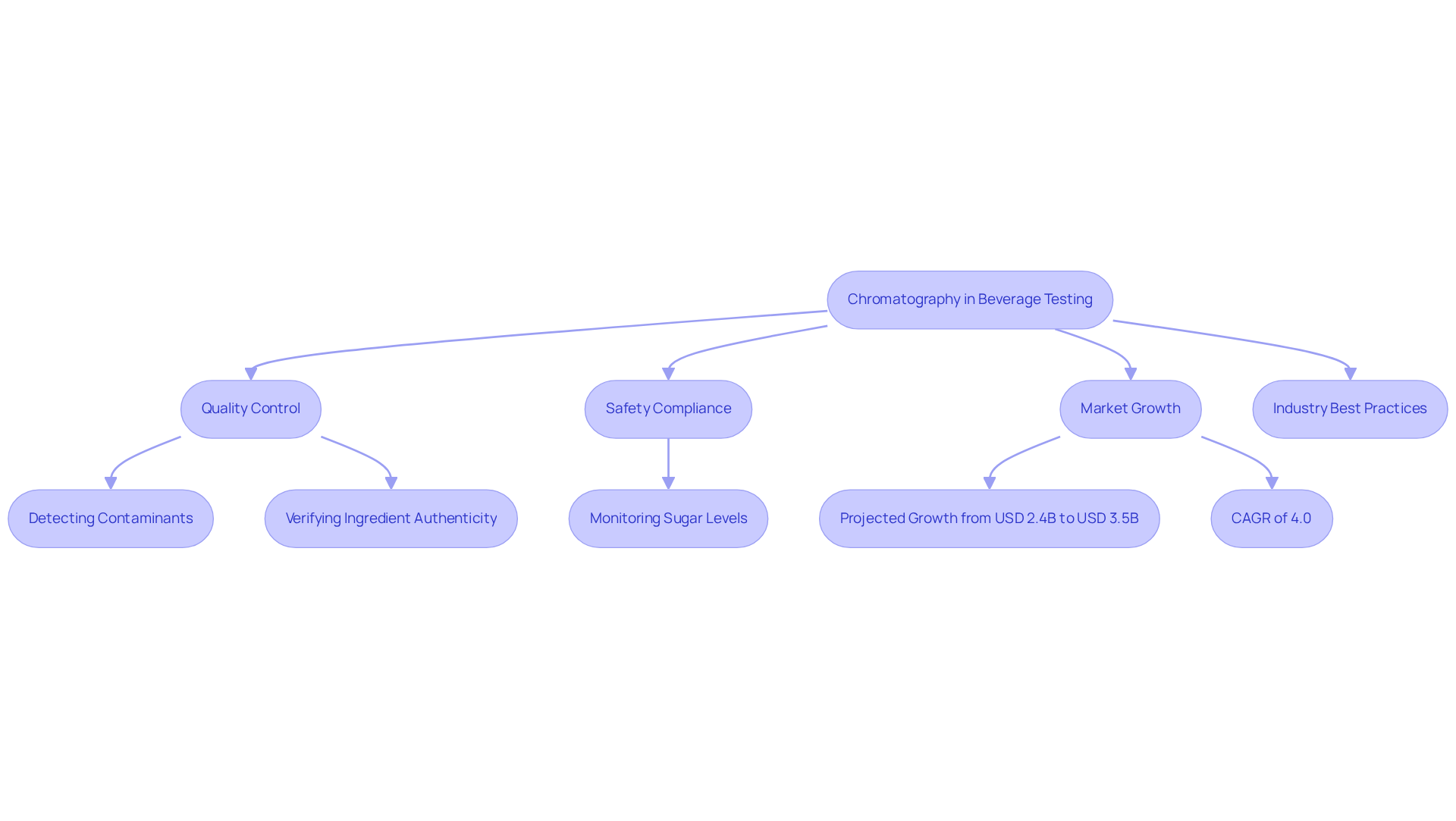
Chromatography applications are essential in beverage testing, playing a pivotal role in ensuring quality and safety. High-performance liquid separation methods are critical for analyzing beverage compositions, enabling manufacturers to detect contaminants and verify ingredient authenticity. This rigorous testing is vital for compliance with health regulations and safeguarding consumer safety.
Current best practices in the industry involve systematically applying separation techniques to monitor various parameters, including sugar levels and potential adulterants. For instance, renowned brands like Jägermeister utilize analytical techniques to ensure flavor uniformity by meticulously tracking sugar levels throughout their products.
Furthermore, the beverage tester market is anticipated to experience significant growth, projected to rise from USD 2.4 billion in 2025 to USD 3.5 billion by 2035, reflecting a compound annual growth rate (CAGR) of 4.0%. This growth underscores the increasing reliance on as separation techniques for quality assurance in the beverage sector.
The real-world chromatography applications of liquid separation techniques go beyond flavor consistency; they also include compliance with safety standards. The 2013 horsemeat scandal exposed the limitations of traditional food analysis methods, prompting a transition towards more precise techniques such as HPLC combined with mass spectrometry (HPLC-MS). This approach proved crucial in validating meat products, demonstrating the broader implications of this technique in maintaining product integrity.
In conclusion, chromatography applications serve not only as tools for quality control but also as vital components in the ongoing efforts to enhance beverage safety and compliance in an increasingly competitive market.
Polymer Analysis: Advancements in Material Science through Chromatography
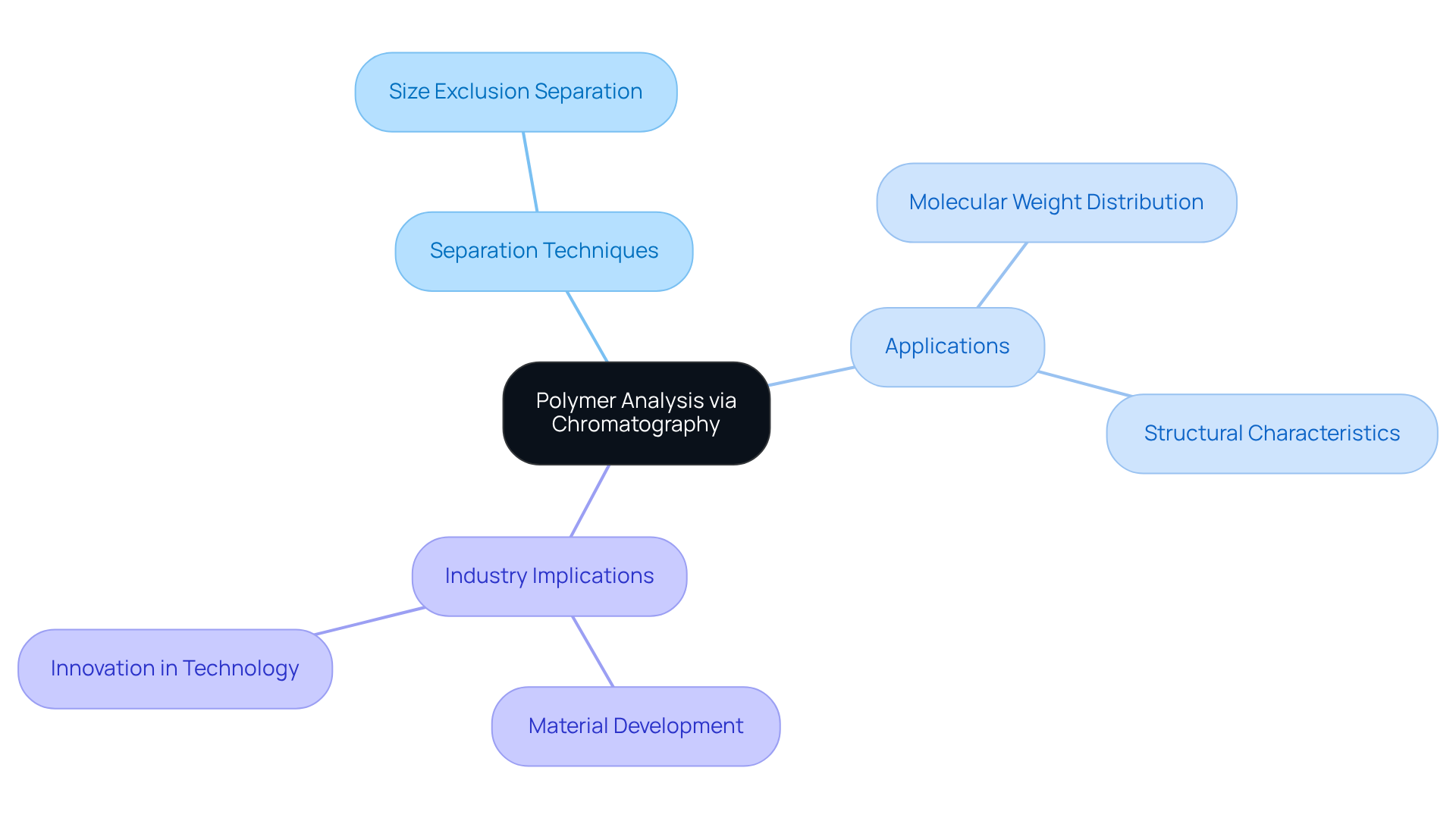
In the field of material science, separation techniques play a crucial role in polymer analysis, enabling researchers to gain insights into their properties and behaviors. Techniques such as size exclusion separation are employed to accurately determine molecular weight distributions and structural characteristics of polymers. This application is not merely a technical detail; it is vital for the development of new materials and the enhancement of existing ones. The implications of these advancements extend across various industries, underscoring the importance of in laboratory settings. By leveraging these techniques, researchers can drive innovation and improve material performance, ultimately contributing to the progress of technology and industry.
Metabolomics: Exploring Biological Processes with Chromatography Techniques
Chromatography applications are essential in metabolomics, which involves the study of metabolites and metabolic processes within organisms. By employing methods such as liquid separation combined with mass spectrometry (LC-MS), researchers can dissect complex biological samples, providing valuable insights into metabolic pathways and disease mechanisms.
JM Science Inc. presents a comprehensive range of premium HPLC solutions, including high-performance liquid chromatography columns and accessories, which are essential for various chromatography applications. Furthermore, our innovative medical devices, such as electronic stethoscopes, facilitate remote patient monitoring, thereby enhancing the capabilities of pharmaceutical laboratories.
This application is essential for deepening our and for the development of new therapeutic strategies.
Conclusion
The importance of chromatography in various scientific fields cannot be overstated, particularly within pharmaceutical labs where precision and accuracy are paramount. This article has highlighted ten essential applications of chromatography, demonstrating how techniques like high-performance liquid chromatography (HPLC) play a critical role in:
- Drug development
- Vaccine formulation
- Food safety
- Forensic science
- Environmental monitoring
- Food safety assessments
- Clinical diagnostics
- Additional applications (to be specified)
By leveraging advanced separation methods, laboratories can ensure the integrity and quality of their products, ultimately safeguarding public health and enhancing scientific research.
Key insights discussed include the pivotal role of chromatography in vaccine safety testing, where rigorous analysis guarantees efficacy and compliance with health standards. Furthermore, the article explored how chromatography aids in various industries, illustrating its versatility. Each application showcases the necessity of high-quality instruments and methodologies in achieving reliable results, reinforcing the core message that chromatography is indispensable in modern scientific endeavors.
As the landscape of research and industry continues to evolve, embracing innovative chromatography techniques will be crucial for maintaining high standards of quality and safety. Laboratories are encouraged to invest in advanced chromatography solutions to enhance their analytical capabilities and remain at the forefront of scientific advancements. The future of chromatography is bright, with significant potential for growth and development in applications that will further contribute to public health, safety, and the advancement of technology.




