Overview
The article delves into the critical understanding of the pH of hydrogen peroxide and its vital role in laboratory applications. It elucidates that the pH of peroxide, generally ranging from 4.5 to 7 based on concentration, is essential for its stability and reactivity. This pH range significantly influences various processes, including disinfection and biochemical assays. Such insights underscore the necessity of precise pH measurement, which is paramount for achieving reliable scientific results. By recognizing the importance of pH in these contexts, laboratory professionals can ensure optimal performance of their experiments and applications.
Introduction
The concept of pH stands as a cornerstone in the realm of chemistry, profoundly influencing the behavior of solutions and the effectiveness of chemical reactions. Defined as the measure of acidity or basicity in aqueous solutions, pH plays a vital role across diverse fields such as agriculture, medicine, and environmental science. With a scale ranging from 0 to 14, where 7 denotes neutrality, understanding pH is essential for optimizing processes like nutrient absorption in crops and ensuring the stability of pharmaceuticals.
Recent advancements in pH measurement technologies, including innovative sensors and automated systems, are transforming how industries monitor and control pH levels. This transformation not only enhances product quality but also ensures regulatory compliance. The significance of pH extends into various applications, particularly focusing on its impact on hydrogen peroxide and laboratory practices. This highlights the critical need for precise pH management in scientific endeavors, underscoring its importance in achieving reliable and accurate results.
Define pH and Its Importance in Chemistry
pH, or 'potential of hydrogen ions,' is a logarithmic scale that quantifies the acidity or basicity of an aqueous solution, ranging from 0 to 14. A pH of 7 is considered neutral, with values below indicating acidity and those above indicating alkalinity. The pH scale is fundamental in chemistry, influencing solubility, nutrient availability, chemical behavior, and reaction rates. Its significance spans multiple areas, such as agriculture, medicine, and environmental science, where it plays a vital role in biological processes and chemical stability.
In analytical applications, particularly in the production of hydrogen peroxide, the pH of peroxide significantly impacts the stability and reactivity of intermediates, thus influencing yield. The integration of advanced technologies, such as Osaka Soda's NQAD, enhances the selectivity and sensitivity of pH measurements, allowing for more accurate detection of components that may have been overlooked in traditional methods. Moreover, exemplifies how precise pH monitoring is essential for compliance with pharmaceutical standards, ensuring the quality and efficacy of drug formulations.
Recent studies underscore the [importance of pH in agricultural practices](https://fortunebusinessinsights.com/ph-sensors-market-110871), where optimal pH levels enhance nutrient uptake in crops, directly impacting yield and quality. In medicine, maintaining appropriate pH levels is vital for patient care, particularly in diagnostics and treatment protocols. The global pH measurement market has experienced substantial growth, with a reported increase of over 15% annually from 2019 to 2023, reflecting the rising demand for precise pH monitoring in these sectors.
As highlighted in recent reports, the integration of modern pH sensors with IoT and automation technologies is revolutionizing how industries monitor and control pH levels, ensuring product quality and regulatory compliance. This evolution in pH measurement technology is crucial for advancing scientific research and applications, reinforcing the pivotal role of pH in chemistry. According to the IMARC Team, 'The report is excellent and has a good amount of data, and our team is extremely happy with the information provided,' emphasizing the significance of accurate pH measurement in laboratory settings.
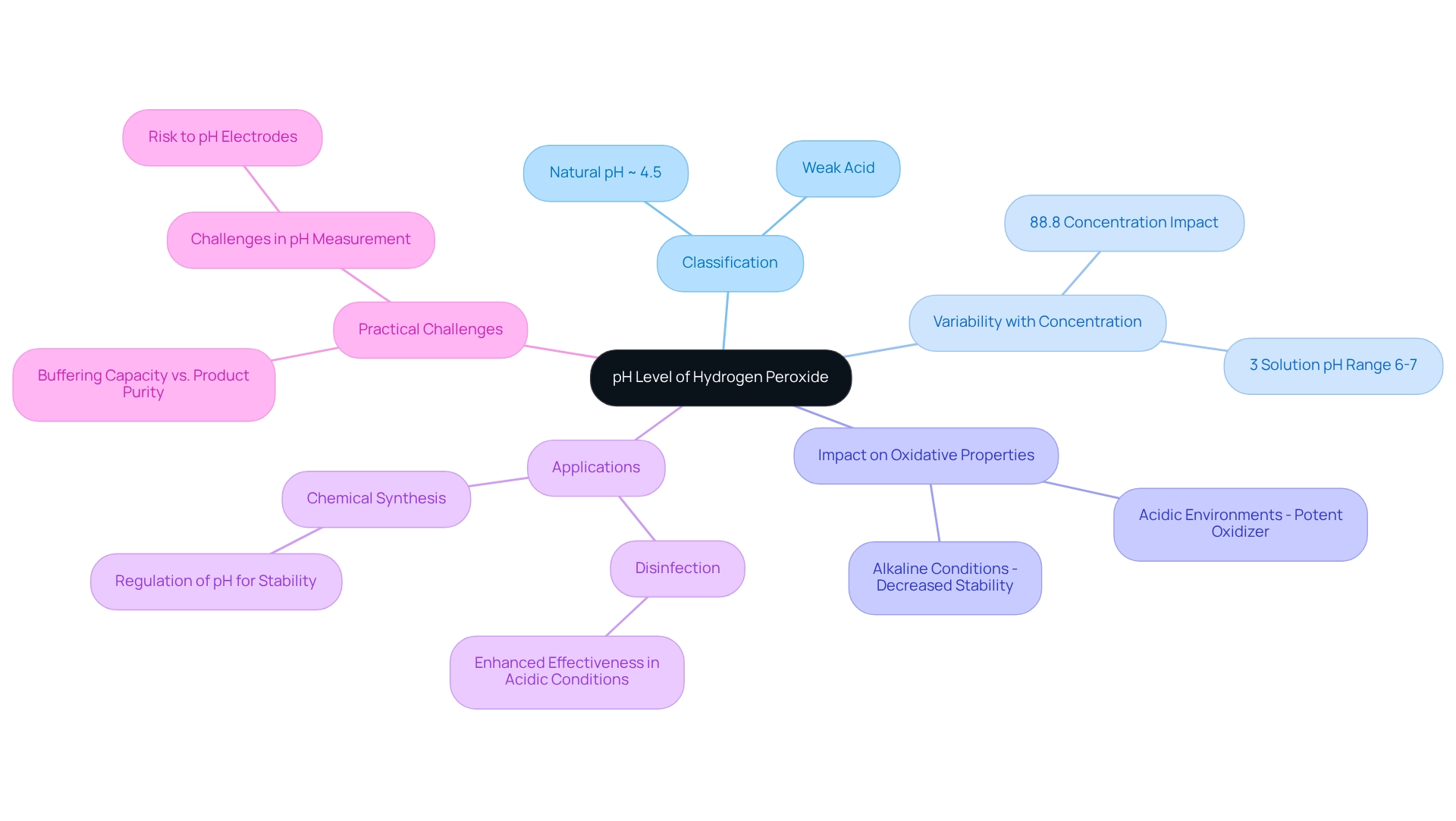
Examine the pH Level of Hydrogen Peroxide
Hydrogen peroxide (H2O2) is typically classified as a weak acid, which has a natural pH of peroxide of approximately 4.5. However, the pH of peroxide can vary significantly based on the concentration and formulation of the solution. For example, a 3% solution of oxygenated water generally displays a pH range of 6 to 7, categorizing it as slightly acidic to neutral. Understanding the pH of peroxide is vital, as it directly impacts its oxidative properties. In acidic environments, H2O2 acts as a potent oxidizer; conversely, in alkaline conditions, its stability decreases, leading to accelerated decomposition.
This pH variability, particularly the pH of peroxide, is crucial in various applications, including disinfection and chemical synthesis. Maintaining the optimal pH of peroxide can enhance the effectiveness of the chemical in sanitizing surfaces, where its oxidative strength is amplified in acidic environments. Conversely, in chemical reactions, precise regulation of the pH of peroxide is essential to ensure its stability and efficacy as a reagent.
Recent studies indicate that the buffering capacity of oxygenated water solutions is inversely related to product purity; solutions of lower purity tend to exhibit greater buffering abilities. This relationship underscores the significance of the pH of peroxide in evaluating the stability and effectiveness of H2O2 in laboratory settings. As of 2025, the current pH of peroxide solutions continues to reflect these dynamics, highlighting the necessity for meticulous monitoring in both research and practical applications.
Moreover, it is noteworthy that the initial concentration of H2O2 in subsequent experiments was determined to be 88.8% by weight, which can significantly influence the pH of peroxide and its behavior across various applications. As Dewan Nirjhor aptly remarked, "And while pH strips are cheap, I don't want to risk my pH electrode in !" This statement emphasizes the practical challenges faced by lab supervisors when measuring pH in oxygenated water solutions.
In conclusion, the pH of peroxide is a critical factor affecting its stability and reactivity, making it imperative for managers to consider this aspect when utilizing this compound in diverse scientific and medical contexts. JM Science's commitment to providing high-performance HPLC components, titrators, and reliable instrumentation fosters advancement in research, further underscoring the importance of accurate pH measurement in experimental environments.
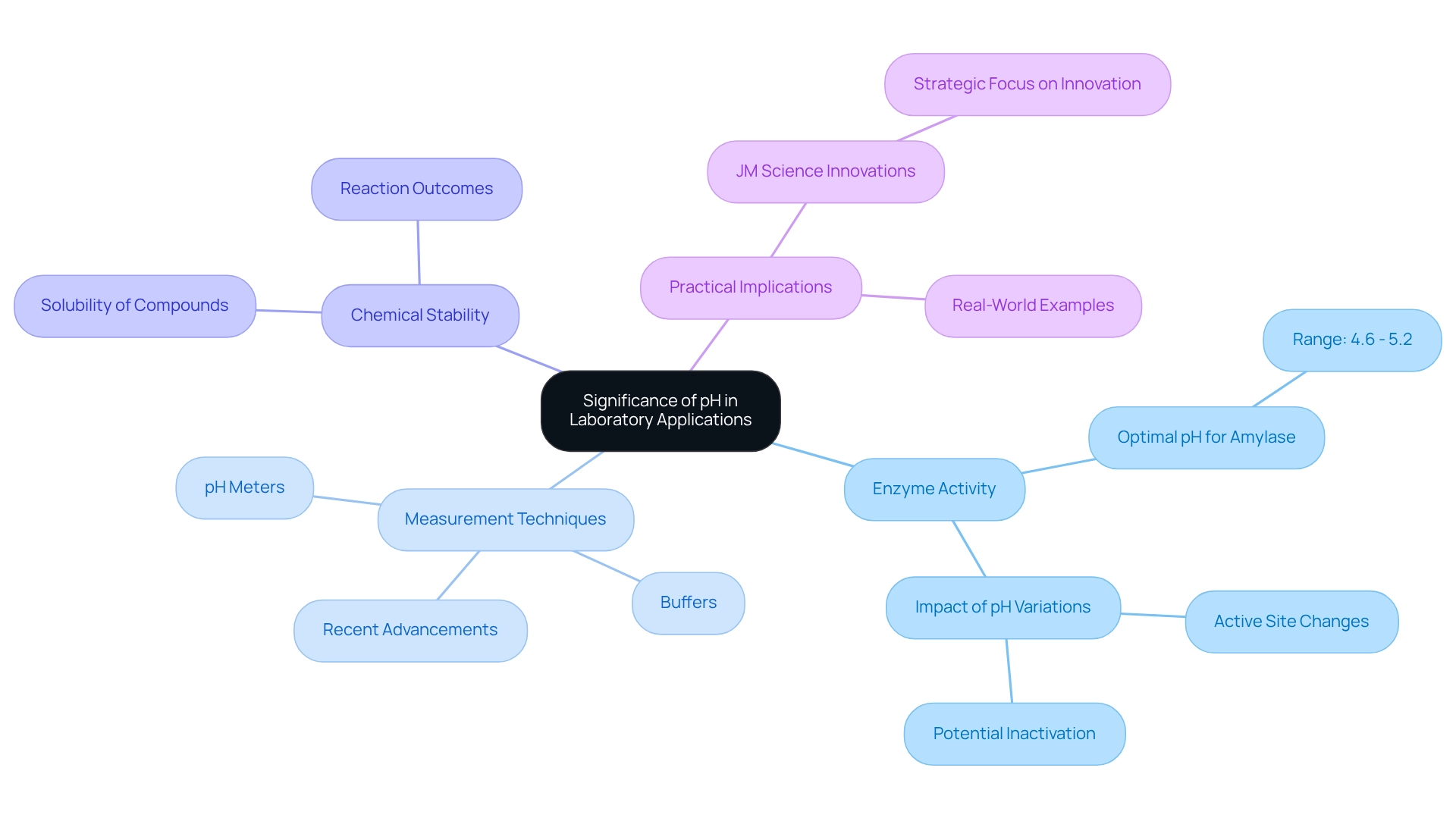
Discuss the Significance of pH in Laboratory Applications
In experimental applications, the pH of peroxide plays a crucial role in various processes, such as titrations, enzyme activity, and chemical stability. Accurate pH measurement, particularly the pH of peroxide, is vital for ensuring that reactions proceed as intended, especially in biochemical assays where enzyme functionality is highly pH-dependent. For instance, many enzymes exhibit optimal activity within specific pH ranges; the optimum pH for Amylase (malt) falls between 4.6 and 5.2. Deviations from this range can lead to diminished activity or even denaturation. Research indicates that the active site of an enzyme may change shape with pH variations, potentially inactivating the enzyme permanently or allowing it to regain functionality if conditions improve.
Furthermore, pH influences the solubility of compounds, which subsequently affects reaction outcomes and product stability. Laboratories commonly employ pH meters and buffers to maintain target pH levels, ensuring reliable and reproducible results across experiments. The equilibrium constants K_EH and K_EOH, which are independent of temperature, underscore the significance of pH in experimental analyses. As noted by Sheryl A Barringer, "The equilibrium constant K_EH and K_EOH (expressed in the p-notation) were found to be independent of temperature." Thus, understanding and controlling the pH of peroxide is fundamental for achieving accurate and meaningful scientific results, especially in the context of enzyme activity and biochemical assays.
Moreover, JM Science Inc. demonstrates a commitment to innovation in , consistently updating its product offerings to enhance scientific practices. Recent advancements in pH measurement tools and methods have significantly improved accuracy and reliability, providing professionals with the resources necessary to achieve optimal results. Real-world examples of pH's effect on enzyme activity in biochemical assays further illustrate the practical implications of pH control, emphasizing its critical role in laboratory applications.
Conclusion
The exploration of pH underscores its fundamental importance across various fields, particularly in chemistry, agriculture, medicine, and environmental science. As a measure of acidity and basicity, pH significantly influences solubility, nutrient availability, and reaction rates, making it essential for optimizing processes in diverse applications. Advancements in pH measurement technologies, such as innovative sensors and automated systems, are revolutionizing how industries monitor and control pH levels, ensuring product quality and regulatory compliance.
In the context of hydrogen peroxide, understanding its pH is critical for effectively harnessing its oxidative properties. The variability of pH in hydrogen peroxide solutions directly impacts stability and reactivity, emphasizing the necessity for precise pH management in both laboratory and practical applications. This is particularly true in disinfection processes and chemical synthesis, where optimal pH levels can enhance efficacy.
Laboratory applications further highlight the significance of pH, as it plays a crucial role in biochemical assays and enzyme activity. Accurate pH measurements are vital for ensuring that reactions proceed as intended, affecting everything from enzyme functionality to product stability. The commitment of companies like JM Science to innovate in pH measurement technology underscores the ongoing need for reliable tools that support scientific research and practical applications.
Ultimately, the critical role of pH in various scientific and industrial contexts cannot be overstated. Understanding and managing pH levels is essential for achieving reliable results and optimizing processes, reinforcing the necessity for ongoing advancements in measurement technologies. As industries continue to evolve, the importance of precise pH management will remain a cornerstone of successful scientific inquiry and application.




