Overview
The article underscores the critical importance of understanding the pKa of citric acid within pharmaceutical applications, emphasizing its pivotal role in influencing drug behavior in biological systems. Knowledge of pKa is not merely beneficial; it is essential for enhancing drug stability, solubility, and efficacy. This understanding directly affects formulation development, buffer preparation, and drug interactions, ultimately leading to improved quality of pharmaceutical products. By grasping the intricacies of pKa, professionals can significantly elevate the effectiveness of their drug formulations, ensuring they meet the rigorous demands of the healthcare industry.
Introduction
Understanding the pKa of citric acid is not merely a niche interest; it stands as a cornerstone of effective pharmaceutical development. This critical parameter acts as a gateway to optimizing drug formulation, influencing essential factors such as solubility and bioavailability. However, the challenge resides in the accurate measurement and application of these pKa values in real-world laboratory settings.
How can pharmaceutical scientists leverage the pKa of citric acid to enhance drug efficacy and stability? This inquiry not only highlights the importance of pKa but also underscores the necessity for precise scientific instruments in laboratory environments.
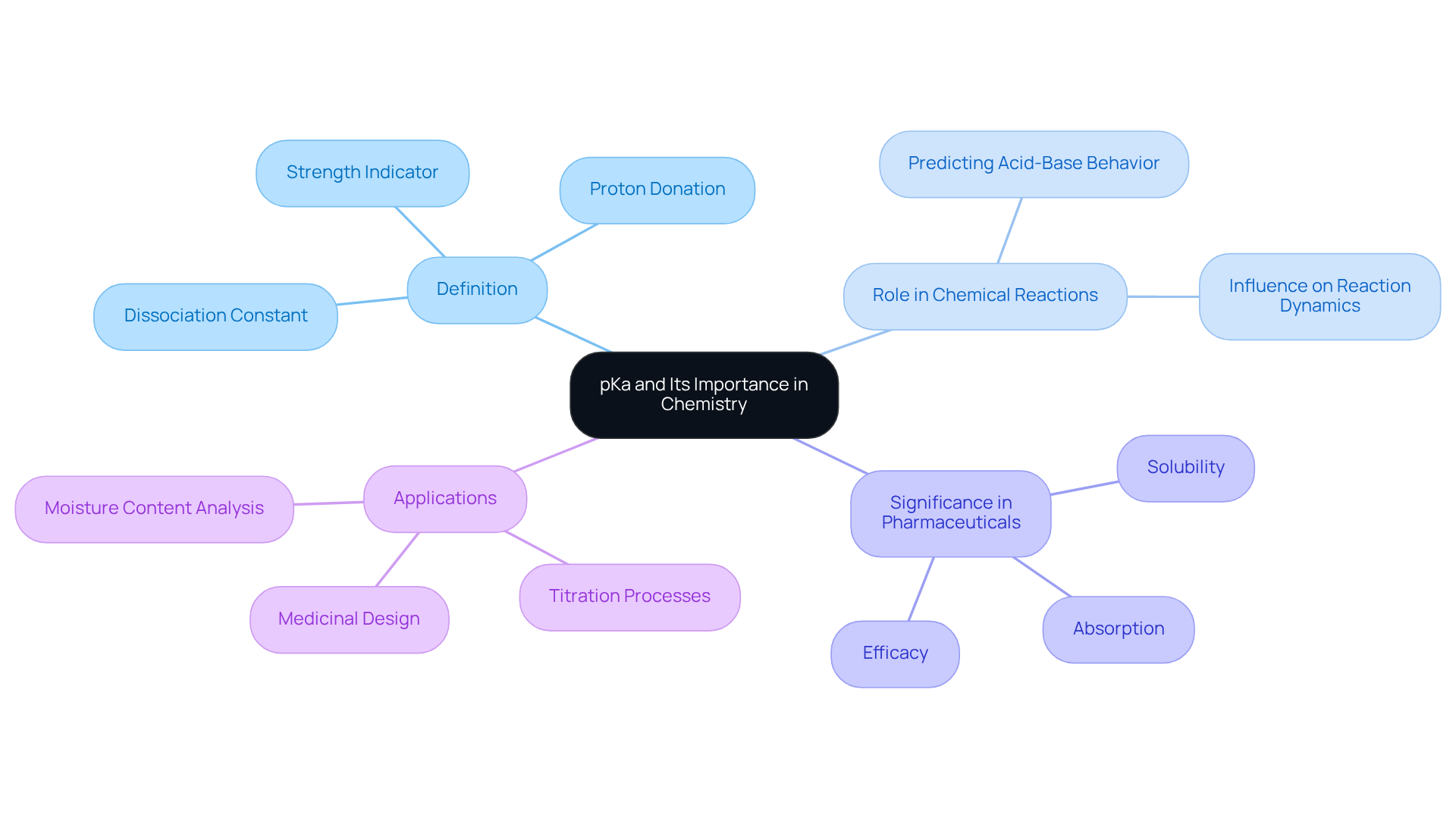
Define pKa and Its Importance in Chemistry
pKa, known as the dissociation constant, serves as a quantitative measure of a substance's strength in solution. It signifies the propensity of a substance to donate protons (H+) to a solution. A lower pKa value indicates a stronger substance, suggesting a more complete dissociation in solution.
Grasping the concept of the pKa of citric acid is crucial in chemistry, as it aids in predicting the behavior of acids and bases across various chemical reactions, particularly within biological systems. In the realm of pharmaceuticals, the pKa of citric acid measurements are vital for evaluating solubility, absorption, and the overall efficacy of pharmaceutical compounds.
For instance, the pKa of citric acid can significantly influence its stability and interactions with biological targets, rendering it a pivotal parameter in medicinal design and formulation. The Hiranuma Aquacounter AQV-300 and AQ-300 titrators leverage pKa values to enhance the precision of moisture content analysis in pharmaceutical formulations, ensuring adherence to the Japanese Pharmacopoeia.
By comprehending the pKa of citric acid, pharmaceutical laboratories can refine their titration processes, ultimately leading to more effective drug testing and development.
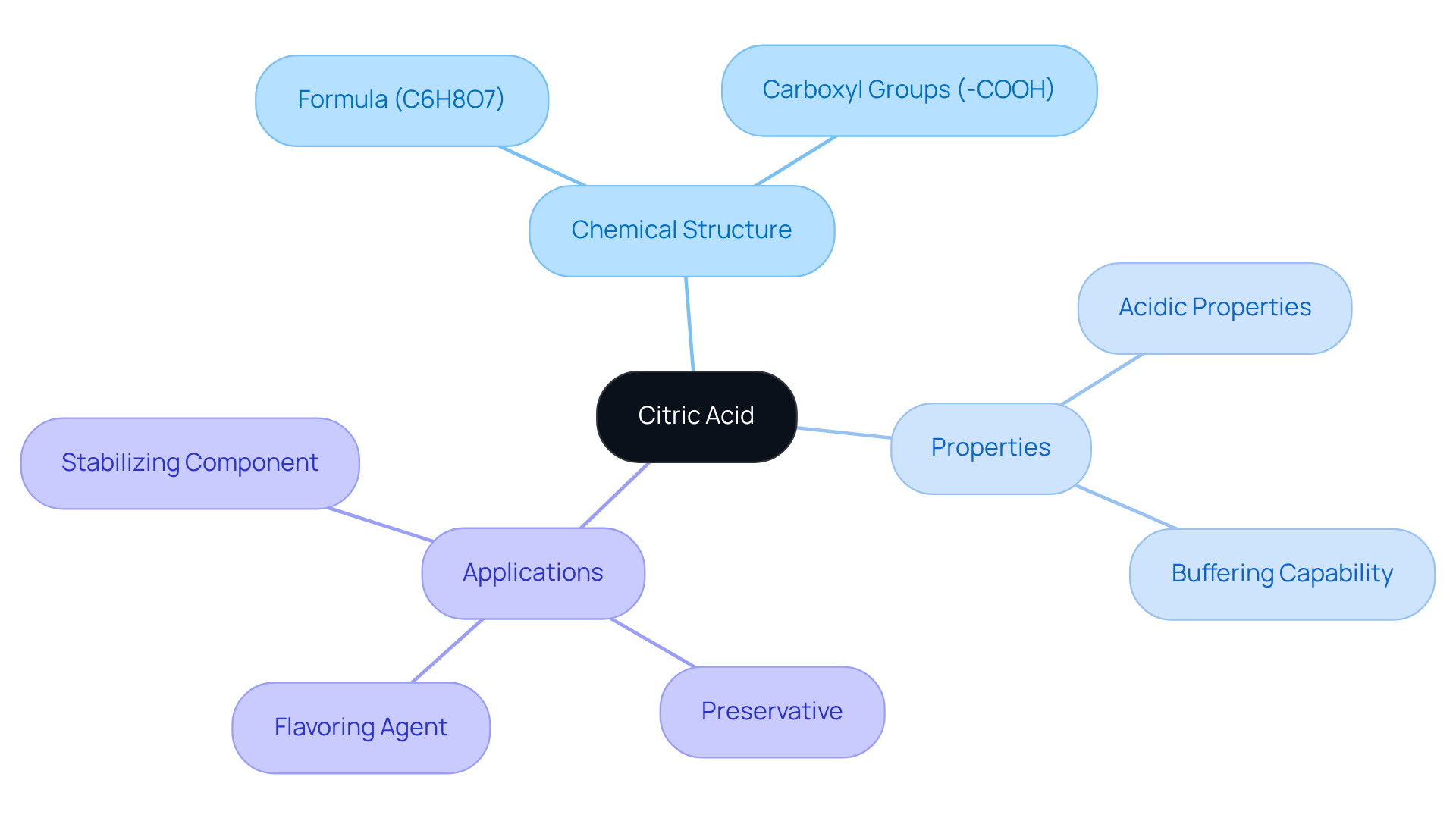
Explore the Structure and Properties of Citric Acid
Citric substance, a weak organic compound with the chemical formula C6H8O7, stands as a vital tricarboxylic compound predominantly found in citrus fruits. Its molecular structure showcases three carboxyl groups (-COOH), which confer its characteristic acidic properties. This presence of multiple acidic protons empowers citric substance to act effectively as a buffer in biological systems, maintaining pH levels within a precise range.
In the realm of pharmaceuticals, citric substance is frequently employed not only as a preservative and flavoring agent but also as a stabilizing component in various formulations. Grasping its molecular structure and properties is essential for predicting its behavior across different environments, particularly concerning the pKa of citric acid.
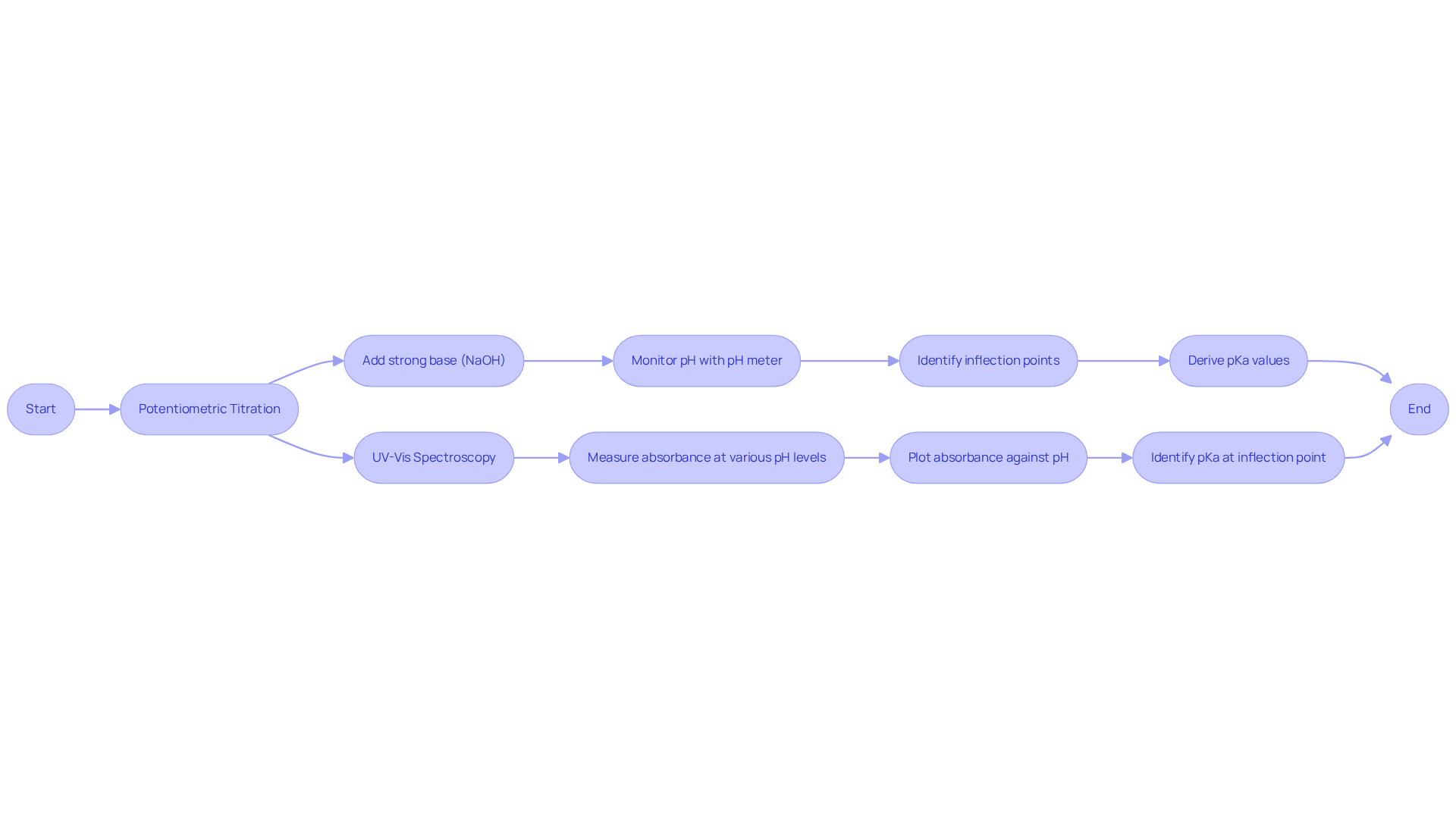
Measure pKa: Techniques and Instrumentation
Determining the pKa of citric acid can be effectively accomplished through various methods, notably potentiometric titration and UV-Vis spectroscopy.
Potentiometric Titration: This technique involves the gradual addition of a strong base, such as sodium hydroxide (NaOH), to a citric solution while continuously monitoring the pH with a pH meter. The pKa values are derived from the inflection points in the titration curve, where a rapid change in pH occurs with the addition of the base. Potentiometric titration is known for its high precision and accuracy, making it a standard approach for pKa measurement. It is essential to use solutions of at least 10-4 M to detect significant changes in the titration curves, thus ensuring reliable results.
UV-Vis Spectroscopy: This method determines pKa by examining the absorbance variations of citric compound across different pH levels. By plotting absorbance against pH, the pKa can be identified at the inflection point of the resulting curve. UV-Vis spectroscopy is particularly advantageous for compounds with lower solubility, as it can , thus providing reliable pKa determinations even in challenging conditions. A UV-active chromophore must be present near the acid-base functional site for effective measurements.
Both methods necessitate meticulous calibration and adherence to standard operating procedures to ensure the accuracy of results. Compliance with regulatory standards is crucial during the measurement process, as it guarantees the reliability of the data obtained. The combination of these techniques not only improves the comprehension of citric substance's behavior in pharmaceutical applications but also highlights the significance of accurate instrumentation in laboratory analyses. As noted in recent studies, the accuracy of potentiometric titration and the sensitivity of UV-Vis spectroscopy are vital for achieving reliable pKa of citric acid measurements, which are essential for the development and formulation of pharmaceutical products.
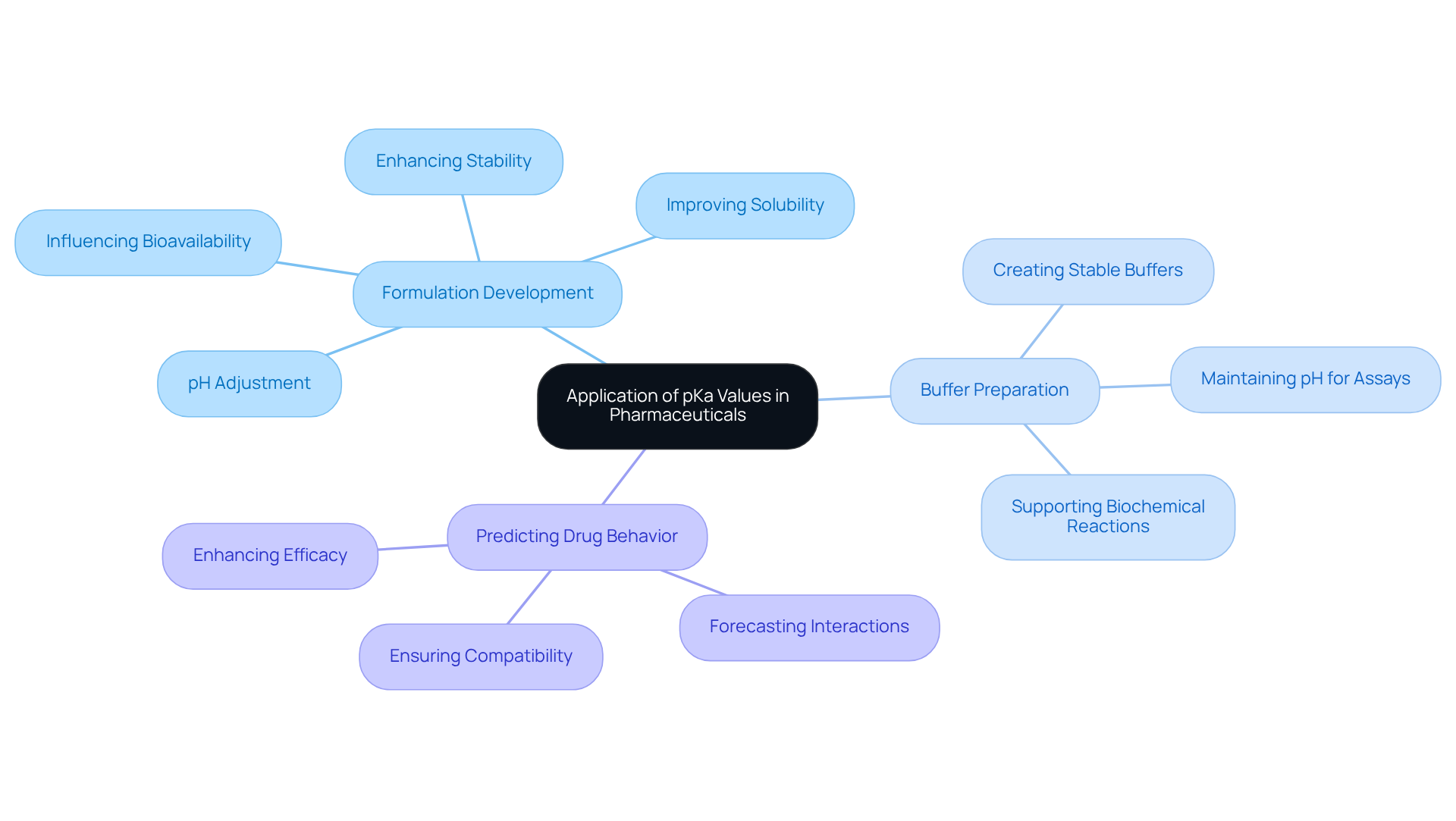
Apply pKa Values in Pharmaceutical and Laboratory Settings
In pharmaceutical and laboratory settings, the application of pKa values is essential for several compelling reasons.
In formulation development, understanding the pKa of citric acid substances plays a crucial role. It assists formulators in adjusting the pH of pharmaceutical solutions, thereby enhancing stability and solubility. For instance, a drug's bioavailability is significantly influenced by its ionization state, which is directly related to the formulation's pH.
- Buffer Preparation: The citric compound is frequently utilized to prepare buffer solutions. A thorough comprehension of its pKa measurements enables researchers to create buffers that maintain a stable pH, which is vital for various biochemical assays and reactions.
- Predicting Drug Behavior: The pKa of citric acid is instrumental in forecasting its interactions with other compounds within a formulation, including active pharmaceutical ingredients (APIs). This knowledge is paramount for ensuring compatibility and efficacy in medicinal products.
By integrating pKa values into laboratory practices, researchers can significantly enhance the quality and effectiveness of their pharmaceutical products, ultimately contributing to successful drug development initiatives.
Conclusion
Understanding the pKa of citric acid is crucial for its effective application in pharmaceuticals, as it directly influences the behavior of this compound in various formulations. By grasping the nuances of pKa, researchers and formulators can predict how citric acid will interact within biological systems, ensuring the stability and efficacy of pharmaceutical products.
This article delves into the significance of pKa in chemistry, highlighting its role in determining the strength of acids and bases, particularly citric acid. Key points include the methods for measuring pKa, such as:
- Potentiometric titration
- UV-Vis spectroscopy
These methods provide accurate results essential for pharmaceutical applications. Furthermore, an understanding of pKa aids in formulation development, buffer preparation, and predicting drug behavior, all of which are vital for successful drug development.
Ultimately, the insights presented underscore the importance of integrating pKa values into laboratory practices. As the pharmaceutical industry continues to evolve, leveraging precise pKa measurements will remain pivotal in enhancing drug solubility, stability, and overall effectiveness. Embracing this knowledge not only streamlines the formulation process but also contributes to the advancement of pharmaceutical science, ultimately benefiting patient care and therapeutic outcomes.




