Overview
This article delves into the critical reaction steps between phosphoric acid and sodium hydroxide, elucidating the stoichiometric relationships and essential safety considerations inherent in this acid-base neutralization process. Understanding the balanced chemical equation is paramount, as it lays the groundwork for accurate reactions in laboratory settings. Moreover, adherence to proper laboratory practices cannot be overstated; these factors are vital for maintaining safety and ensuring reliable outcomes in scientific investigations. By grasping these concepts, researchers can navigate the complexities of chemical interactions with confidence and precision.
Introduction
In the realm of chemistry, the interaction between phosphoric acid and sodium hydroxide exemplifies acid-base neutralization, showcasing the intricate dance of protons and hydroxide ions. This reaction transforms these two compounds into sodium phosphate and water, while also unlocking a wealth of applications across various industries, from pharmaceuticals to sustainable practices.
Understanding the properties, reaction mechanisms, and safety protocols associated with these substances is vital for laboratory professionals who aim to enhance operational efficiency and ensure safe handling.
This article delves into the essential aspects of phosphoric acid and sodium hydroxide, providing a comprehensive guide to their properties, the stoichiometry of their reactions, and the necessary precautions to take when conducting experiments.
Explore the Properties of Phosphoric Acid and Sodium Hydroxide
Phosphoric substance (H₃PO₄) is a colorless, odorless liquid or crystalline solid, characterized by a melting point of 42.35 °C and a density of 1.834 g/cm³. As a triprotic acid, it can donate three protons (H⁺ ions) in solution, which plays a crucial role in its reactivity with bases. In contrast, sodium hydroxide (NaOH) is a white, crystalline solid that is highly soluble in water, producing a strong alkaline solution. It has a melting point of 318 °C and is known for , making it essential in various chemical processes, particularly neutralization activities.
Understanding these properties is vital for the safe handling of these substances in laboratory environments. Recent studies have highlighted the importance of these compounds in chemical reactions, showcasing their applications in fields such as pharmaceuticals and industrial processes. For instance, the efficient application of caustic soda in zinc recovery methods has demonstrated recovery rates of up to 34% at 40% solids, with the zinc deposit acquired from electrodeposition achieving values up to 99% purity. This underscores the significance of lye in sustainable practices. Furthermore, the case study titled "Innovative Approaches to Zinc Recovery from Industrial Waste" illustrates innovative hydrometallurgical methods that enhance while minimizing environmental impact.
Safety protocols are essential when working with these substances, as both phosphoric acid and sodium hydroxide can pose hazards if not managed properly. Proper personal protective equipment (PPE) is essential, and adherence to safety guidelines is crucial to mitigate risks. As E.d.l.T. noted, "The research presented in this study was made possible by the financing of the Department of Extractive Metallurgy (DEMEX) of the Escuela Politécnica Nacional thanks to the research project PVIF-20-01," highlighting the collaborative efforts in advancing the understanding of these chemicals. By staying informed about the latest studies and professional insights on the characteristics and uses of phosphoric compounds and alkali substances, laboratory experts can enhance their operational effectiveness and ensure compliance with safety regulations.
Analyze the Reaction Mechanism and Stoichiometry
The interaction between phosphoric acid and sodium hydroxide exemplifies a fundamental acid-base neutralization process. The balanced chemical equation for this reaction is as follows:
H₃PO₄(aq) + 3NaOH(aq) → Na₃PO₄(aq) + 3H₂O(l).
Here, one mole of phosphoric acid interacts with three moles of alkali, resulting in the formation of one mole of sodium phosphate and three moles of water. This stoichiometric relationship underscores how hydroxyl ions (OH⁻) from the sodium hydroxide effectively neutralize protons (H⁺) from the phosphoric acid and sodium hydroxide, leading to the production of water and a salt.
Understanding this stoichiometry is vital for accurately calculating concentrations and evaluating the effectiveness of the neutralization process. In a titration scenario, for instance, knowing the molarity of the NaOH solution enables the calculation of moles of NaOH, which can then be correlated with the moles of phosphoric acid and sodium hydroxide to determine its concentration.
This principle holds particular importance in laboratory settings, especially within pharmaceutical applications where precision is paramount. Practical applications of this stoichiometric relationship manifest in various laboratory techniques, notably titrations used to determine the concentration of phosphoric acid and sodium hydroxide in solutions.
JM Science Inc. offers high-quality titrators, including potentiometric titrators and , which significantly enhance the accuracy and efficiency of these titration processes. For example, the utilization of JM Science's advanced titrators facilitates precise endpoint detection, thereby minimizing errors in concentration calculations.
By comprehending the interaction mechanism and stoichiometry of phosphoric acid and sodium hydroxide, laboratory professionals can better anticipate the behavior of these reactants, optimize their application in experiments, and contribute to advancements in analytical chemistry. JM Science plays a crucial role in this domain by providing premium scientific instruments that support these essential laboratory processes.
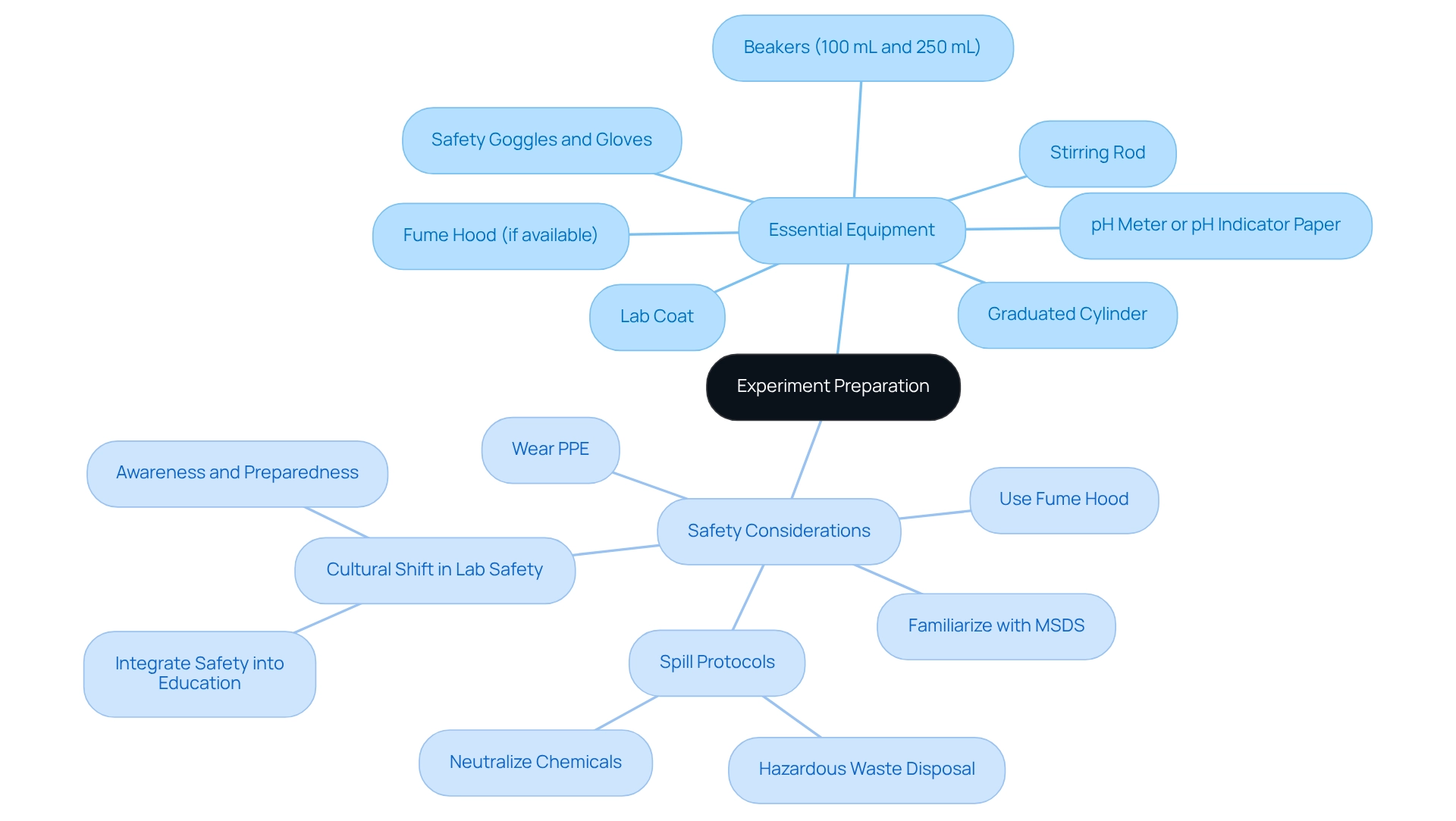
Prepare for the Experiment: Equipment and Safety Considerations
Before conducting the reaction, it is imperative to have the following essential equipment ready:
- beakers (100 mL and 250 mL)
- a graduated cylinder
- a stirring rod
- a pH meter or pH indicator paper
- safety goggles and gloves
- a lab coat
- a fume hood if available
When handling phosphoric acid and sodium hydroxide, safety considerations are paramount. Always wear appropriate personal protective equipment (PPE), including gloves and goggles, to prevent skin and eye contact. Statistics indicate that proper PPE usage significantly reduces the risk of injury in laboratory settings. It is advisable to work in a well-ventilated area or utilize a fume hood to minimize exposure to harmful vapors.
In the event of spills, neutralize the chemicals with a suitable absorbent material and adhere to established hazardous waste disposal protocols. Familiarize yourself with the Material Safety Data Sheets (MSDS) for phosphoric acid and sodium hydroxide to understand their risks and necessary first aid actions. Recent advancements in laboratory safety protocols emphasize the integration of safety into the educational framework for future researchers, ensuring they are well-equipped to conduct safe science. As highlighted in a case study on lab safety culture, improving safety necessitates a cultural shift within academic institutions. Furthermore, advanced solutions from to deliver safer products, reinforcing the importance of using cutting-edge equipment and protocols during chemical reactions. As Dr. Alice Parker, a safety officer, notes, "Chemical labels are our first line of defense; they empower us to act wisely around hazardous materials." This underscores the significance of awareness and preparedness in laboratory environments.
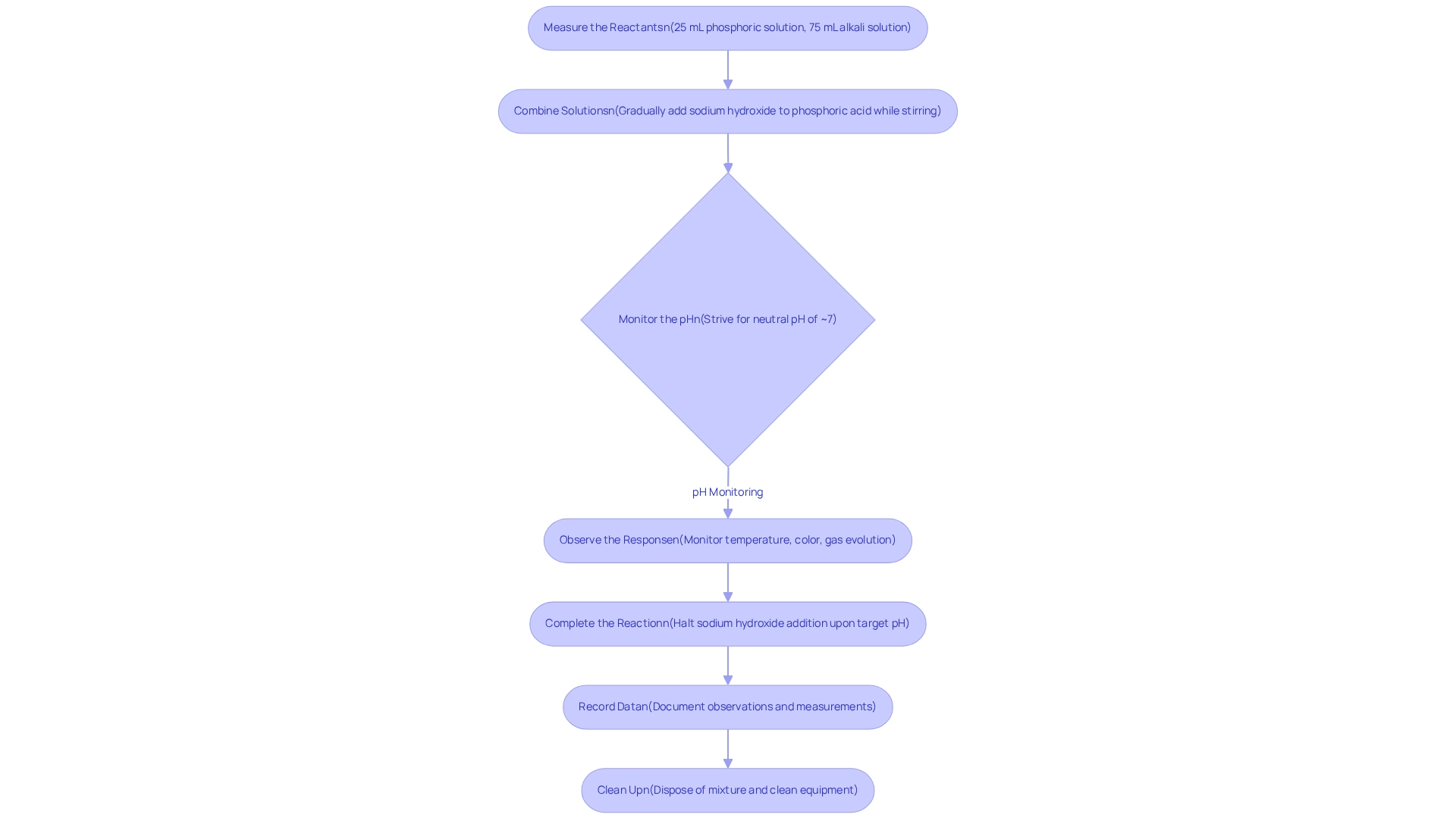
Conduct the Reaction: Step-by-Step Procedure
- Measure the Reactants: Begin by accurately measuring 25 mL of phosphoric solution and 75 mL of alkali solution using a graduated cylinder.
- Combine the solutions by gradually introducing the sodium hydroxide solution to the phosphoric acid and sodium hydroxide mixture in a 250 mL beaker while stirring continuously. This methodical addition is essential to regulate the process rate and minimize excessive heat generation.
- Monitor the pH: Employ a pH meter or pH indicator paper to keep track of the solution's pH. Strive for a neutral pH of approximately 7, which signifies complete neutralization. As emphasized by J. M. Williams, accurate on-site measurements are vital for ensuring reliable results in laboratory environments.
- Observe the Response: Diligently monitor any changes in temperature, color, or gas evolution throughout the reaction.
- Complete the Reaction: Upon reaching the target pH, halt the addition of sodium hydroxide. If necessary, allow the solution to cool.
- Record Data: Meticulously document all observations and measurements for future analysis. Recent studies indicate that during acid-base processes significantly enhances the reliability of results, aligning with the increasing investment in chemical raw materials and product manufacturing, which has surged by 17.8% annually.
- Clean Up: Dispose of the mixture according to your institution's hazardous waste disposal guidelines and ensure all equipment is thoroughly cleaned. Recent experiments have underscored the importance of accurate pH monitoring during acid-base interactions. Studies suggest that precise on-site measurements can greatly enhance result reliability. For instance, selecting appropriate indicators is crucial, as demonstrated in a case study on indicator selection for titrations, which highlights the necessity for indicators that change color at the correct pH level to signal the completion of the reaction. This meticulous approach not only guarantees accurate results but also adheres to best practices in laboratory settings.
Conclusion
The interaction between phosphoric acid and sodium hydroxide exemplifies a fundamental acid-base neutralization reaction, carrying substantial implications across various fields. A thorough understanding of these compounds—encompassing their reactive behaviors and diverse applications—enables laboratory professionals to optimize operational efficiency while strictly adhering to safety protocols. The intricate stoichiometry of this reaction underscores the critical relationship between the reactants, highlighting the necessity of precise measurements to achieve desired outcomes, particularly within pharmaceutical and industrial contexts.
Safety remains an essential priority when conducting experiments involving these chemicals. The use of appropriate personal protective equipment and strict compliance with established safety guidelines are vital to mitigate risks associated with their handling. Cultivating a culture of safety within the laboratory, alongside the utilization of advanced equipment, not only fosters a safer working environment but also empowers researchers to conduct their experiments with assuredness.
In summary, the meticulous study and application of phosphoric acid and sodium hydroxide not only enhance scientific comprehension but also contribute to sustainable practices across industries. By prioritizing safety and precision, professionals can fully leverage the potential of these chemicals, paving the way for innovations that benefit both the scientific community and society as a whole.




