Overview
Anhydrous sodium sulfate stands as a pivotal compound, exhibiting essential properties and a wide range of applications across various industries, including detergents, glass manufacturing, textiles, and pharmaceuticals. Its significance is underscored by high solubility, hygroscopic characteristics, and its vital role as a drying agent. Furthermore, the discussion encompasses market trends, safety guidelines, and the imperative for regulatory compliance, all aimed at minimizing environmental impact. This multifaceted approach not only highlights the compound's utility but also emphasizes the responsibility that comes with its use.
Introduction
In the realm of industrial chemistry, anhydrous sodium sulfate emerges as a multifaceted compound with a vast array of applications across diverse sectors. Renowned for its remarkable solubility and drying capabilities, this white crystalline solid is essential in industries ranging from detergent manufacturing to pharmaceuticals.
As global production trends and market demands evolve, it becomes increasingly crucial to comprehend the composition, applications, and safety protocols associated with anhydrous sodium sulfate. This article explores the chemical properties, manufacturing processes, and market dynamics of this vital compound, illuminating its significance in the ever-evolving landscape of industrial applications.
Understanding Anhydrous Sodium Sulfate: Definition and Composition
Anhydrous sodium sulfate (Na2SO4), commonly referred to as thenardite, is a white crystalline solid devoid of water molecules. This compound is distinguished by its high solubility in water and its efficacy as a drying agent in various chemical processes. Formed when it loses its water of hydration, anhydrous sodium sulfate is integral to numerous industrial applications, particularly within the detergent and glass manufacturing sectors.
The chemical composition of dry sodium salt comprises two Na+ ions and one SO4^2- ion. This unique arrangement contributes to its distinctive properties, establishing it as a vital component in various formulations. Recent studies have underscored its significance in industrial processes, showcasing its versatility in applications that range from detergent production to enhancing the quality and durability of glass products in the glass industry.
The global output of anhydrous sodium sulfate has experienced fluctuations, influenced by economic conditions and regional demands. For instance, North America and Europe have witnessed consistent growth, whereas Latin America and the Middle East and Africa are projected to exhibit slower growth in the chemical sector. This trend highlights the necessity of adapting strategies to address the evolving needs of the industry.
A 2010 report revealed that the global chemical industry value varied significantly by region, emphasizing the diverse landscape of demand and supply.
Despite recent challenges, such as those posed by the COVID-19 pandemic, the demand for anhydrous sodium sulfate has demonstrated resilience. A thorough analysis of the pandemic's impact indicates a range of outcomes, from optimistic to neutral, providing stakeholders with valuable insights for strategic planning. As noted in the case study titled "Impact of COVID-19 on the Market," understanding these dynamics is essential for navigating future challenges.
In summary, the chemical characteristics and industrial significance of this crucial compound across various sectors reinforce its role as a key player in the global chemical industry. As Manoj Phagare, Senior Research Analyst at Cognitive Market Research, asserts, "Evidence-based strategies are essential for fueling the success of key business initiatives in this evolving landscape." JM Science Inc. remains committed to updating its product offerings and fostering strong relationships with leading manufacturers, ensuring it meets the changing needs of the scientific community and contributes to advancements in research and industrial applications.
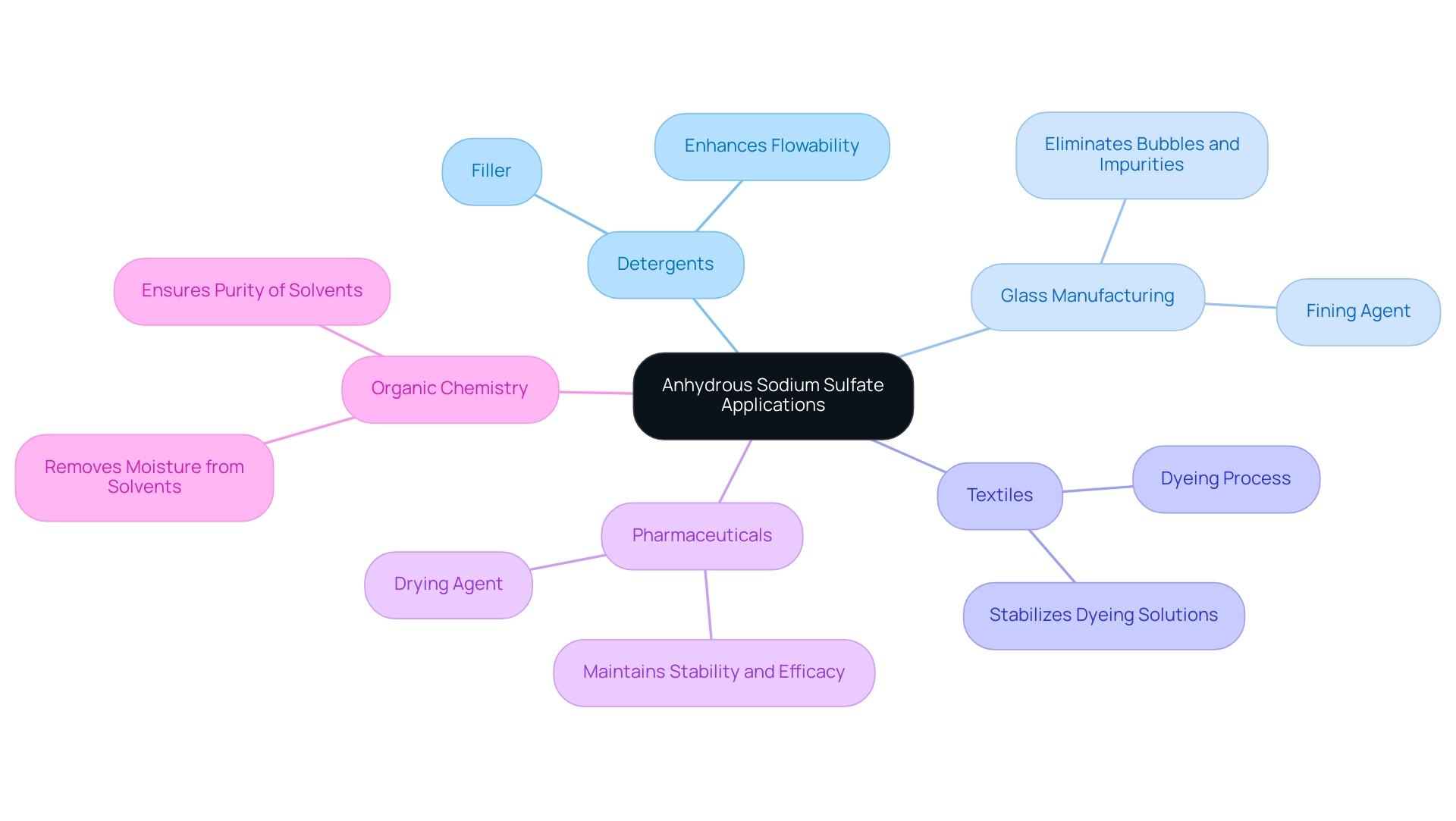
Key Applications of Anhydrous Sodium Sulfate in Industry
Anhydrous sodium sulfate is a versatile compound utilized across multiple industries, each benefiting from its unique properties. In the production of powdered detergents, anhydrous sodium sulfate acts as a crucial filler, enhancing flowability to ensure effective mixing and performance during washing. The demand for salt in this sector is substantial, with a significant portion linked to its role in improving product efficiency. The salt market size surpassed USD 955 million in 2020 and is projected to expand at a CAGR of 4.4% until 2027, driven by increasing applications in the textile sector and growing paper manufacturing.
This compound also serves as a vital fining agent in glass manufacturing. By assisting in the elimination of bubbles and impurities from molten glass, anhydrous sodium sulfate contributes to the creation of high-quality glass products. Its application in this industry is essential for achieving clarity and durability in glass items.
In the textile industry, anhydrous sodium sulfate is employed in dyeing processes, ensuring even distribution of dyes and stabilizing dyeing solutions. This application is critical for achieving consistent color results in textiles, making it a staple in the industry. The Asia Pacific region is anticipated to exhibit significant expansion in the sodium-based salt market, propelled by rising demand for detergents from the industrial sector.
In pharmaceuticals, dry sodium sulfate is utilized as a drying agent during the preparation of various compounds. Its ability to effectively remove moisture is vital for maintaining the stability and efficacy of pharmaceutical products, playing a key role in drug formulation. Furthermore, in organic chemistry, anhydrous sodium sulfate is frequently used as a dry compound to remove moisture from organic solvents after extraction. This step is crucial for ensuring the purity of the solvents, which is essential for accurate experimental results.
The increasing need for dehydrated sodium compounds is evident in industry trends, with the chemical sector size surpassing USD 955 million in 2020 and expected to expand at a CAGR of 4.4% until 2027. This growth is driven by expanding applications in industries such as textiles and detergents, underscoring the compound's importance in modern manufacturing processes. However, it is important to note that Latin America and the Middle East & Africa are expected to demonstrate slow growth in the chemical sector.
As Prem Kumar observed, 'I sincerely value the contributions of my team and am thankful to our clients for their ongoing trust,' highlighting the significance of market insights in comprehending the relevance of dry sodium sulfate across various applications.
Safety and Handling Guidelines for Anhydrous Sodium Sulfate
When working with dry chemical compounds, adhering to strict safety protocols is essential to safeguard the health of laboratory staff. Key recommendations include:
- Personal Protective Equipment (PPE): Wearing suitable PPE, including gloves, safety goggles, and dust masks, is vital. These measures prevent inhalation of dust and skin contact, thereby minimizing health risks associated with exposure.
- Ventilation: Work areas must be well-ventilated to reduce dust accumulation and limit exposure. Sufficient airflow is particularly crucial in confined areas, ensuring a secure working environment.
- Storage: Anhydrous sodium sulfate should be stored in a cool, dry place, away from incompatible materials such as strong acids and oxidizers. Proper storage conditions are necessary to prevent chemical reactions that could lead to hazardous situations.
- Spill Management: In the event of a spill, it is imperative to avoid generating dust. Utilize appropriate methods for collection and disposal, adhering to local regulations to ensure environmental safety.
- Emergency Procedures: All personnel must be familiar with emergency procedures, including first aid measures for exposure. Rapid access to these procedures can significantly mitigate the impacts of unintentional exposure.
Statistics indicate that workplace exposure to chemical compounds may affect over a million employees in the U.S., underscoring the importance of these safety precautions. Furthermore, the maximum allowable concentration of salt in drinking water is 500 mg/L, which highlights the necessity for strict safety protocols. O'Neil MJ notes that a type of salt occurs in nature as the minerals mirabilite, thenardite, and astrakanite, providing context about its natural properties.
Case studies detailing chemical methods of rock analysis emphasize the necessity of accurate measurement and dilution techniques, which are integral to maintaining safety and precision in laboratory settings. By applying these guidelines, laboratories can enhance safety and ensure compliance with health regulations while effectively managing the risks associated with anhydrous sodium sulfate.
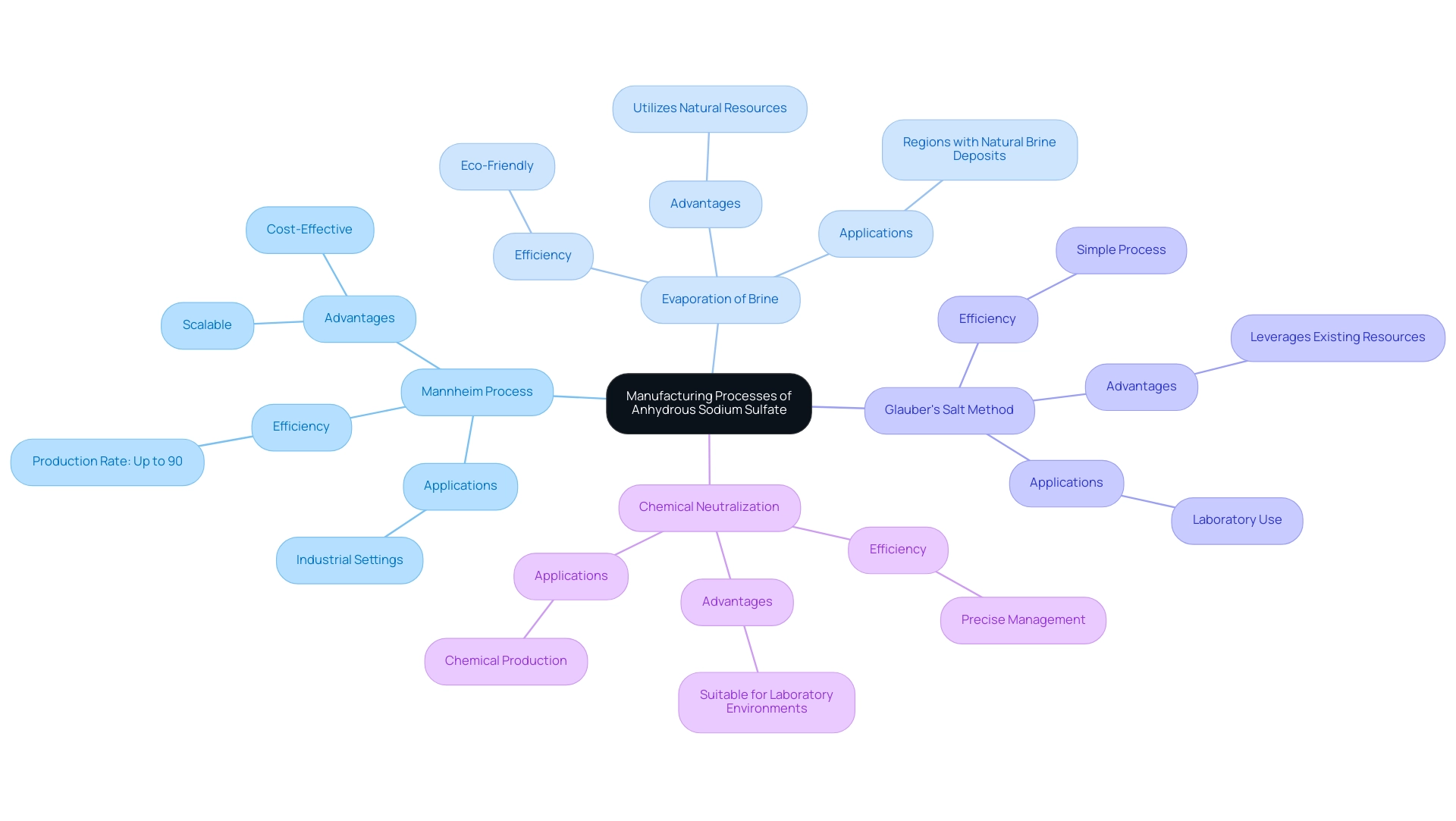
Manufacturing Processes of Anhydrous Sodium Sulfate
Anhydrous sodium salt is produced through several key methods, each characterized by distinct processes and efficiencies.
The Mannheim Process is a widely utilized method that involves the reaction of sodium salt with sulfuric acid, yielding sodium salt as a primary product. This process is notable for its efficiency, achieving production rates of up to 90% under optimal conditions. Its scalability and cost-effectiveness make it particularly favored in industrial settings.
Another common technique is the Evaporation of Brine, which involves the evaporation of natural brine sources. As the brine evaporates, salt crystallizes and is subsequently dehydrated to achieve the dry form. This eco-friendly approach is frequently employed in regions abundant in natural brine deposits.
The Glauber's Salt Method utilizes Glauber's salt (sodium sulfate decahydrate) as its starting material. By heating Glauber's salt, water is eliminated, resulting in dry sodium salt. This method is advantageous due to its simplicity and the ability to leverage existing salt resources.
In the Chemical Neutralization method, sodium hydroxide is neutralized with sulfuric acid to produce salt. The resulting product undergoes further processing to eliminate water, yielding anhydrous sodium sulfate. This method is particularly beneficial in laboratory environments, where precise management of chemical reactions is crucial.
Recent research highlights the adaptability of sodium-based salts, noting their inclusion in a variety of consumer items, such as processed foods and household cleaning agents. A specific type of salt is naturally present in food products and is often included to enhance the texture and stability of processed goods. According to O'Neil MJ, this salt occurs in nature as the minerals mirabilite, thenardite, and astrakanite.
The widespread application of these salts underscores the importance of efficient production methods, with the Mannheim process remaining a cornerstone of sodium-based compound manufacturing.
Expert insights indicate that advancements in production techniques are continuously evolving. Ongoing research aims to enhance the efficiency and environmental impact of these processes. As the demand for high-purity salt escalates, producers are increasingly focusing on innovative techniques that not only improve yield but also minimize waste and energy consumption. JM Science Inc. exemplifies this commitment to innovation by consistently updating its product offerings and fostering strong relationships with leading manufacturers, ensuring they meet the changing needs of the scientific community.
Moreover, the extensive application of certain salts in household items and their identification in soil samples, with a sample size of 5 g, further emphasize their significance in both consumer uses and environmental assessment.
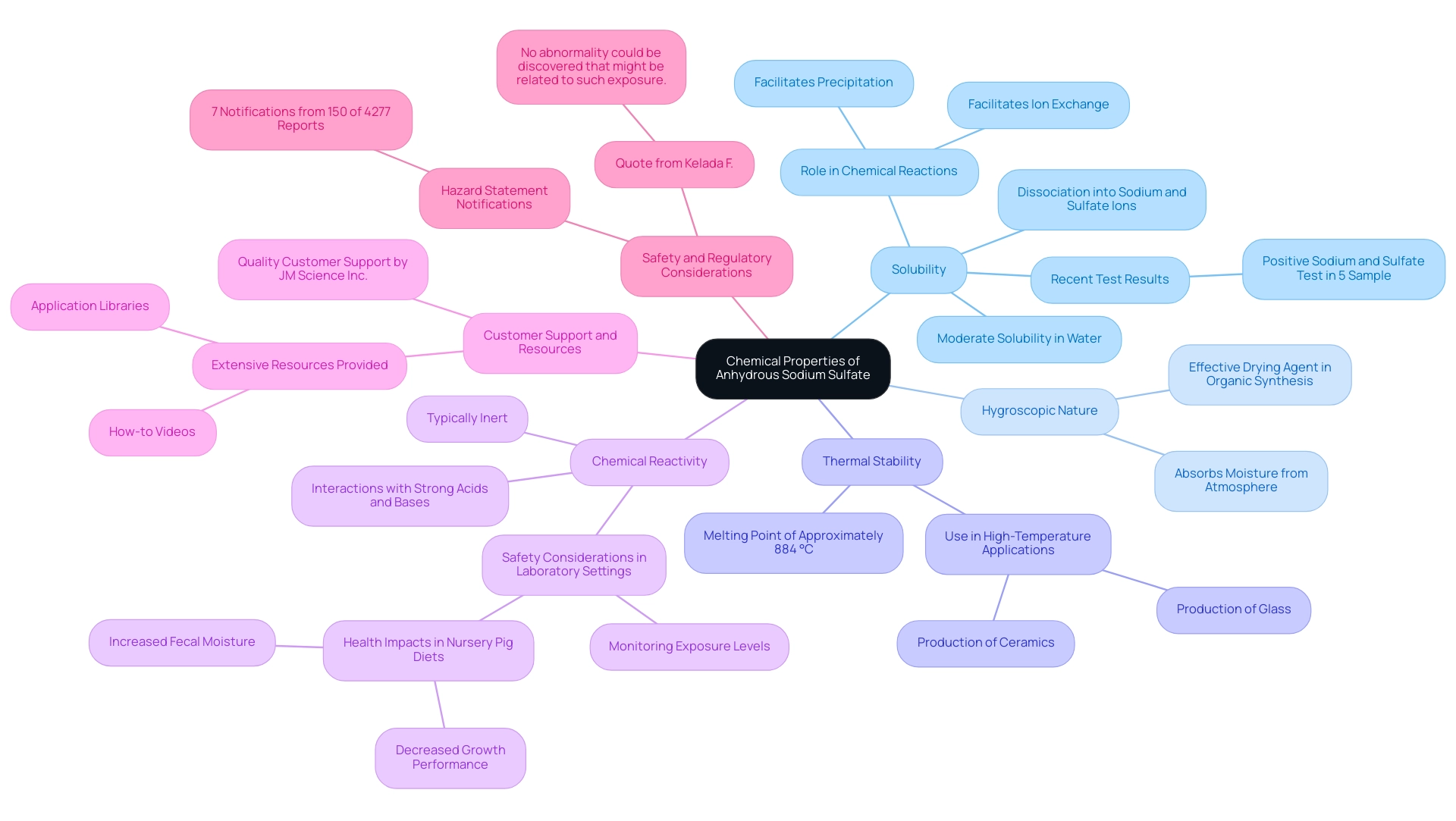
Chemical Properties of Anhydrous Sodium Sulfate: A Detailed Overview
Anhydrous sodium sulfate is characterized by several significant chemical properties that underscore its utility in various applications.
- Solubility: This compound demonstrates moderate solubility in water, dissociating into sodium and sulfate ions. This property is essential for its involvement in numerous chemical reactions, facilitating processes such as precipitation and ion exchange. Recent tests confirmed the presence of a salt compound in a 5% sample liquid, reinforcing its functionality in chemical processes.
- Hygroscopic Nature: Anhydrous sodium sulfate possesses a hygroscopic quality, enabling it to absorb moisture from the atmosphere. This characteristic makes it an effective drying agent in organic synthesis, where the crucial removal of water enhances reaction efficiency.
- Thermal Stability: With a melting point of approximately 884 °C (1623 °F), this dry salt displays exceptional thermal stability. This property enables its use in high-temperature applications, such as in the production of glass and ceramics, where consistent performance under heat is required.
- Chemical Reactivity: While typically regarded as inert, dry salts can interact with strong acids and bases. This reactivity is vital for laboratory settings, where careful consideration of chemical interactions is necessary to ensure safety and accuracy in experiments.
However, it is important to note that the salt compound has been shown to negatively affect health, particularly in nursery pig diets, where it decreased growth performance and increased fecal moisture. This highlights the need for careful consideration of its use in various applications.
Furthermore, specialist views emphasize the significance of comprehending the solubility information of dry salt in water, as it directly affects its use in pharmaceutical formulations and other scientific efforts. The ability of anhydrous sodium sulfate to maintain stability and reactivity under varying conditions makes it a valuable asset in both research and industrial applications, further solidifying its relevance in the scientific community. As noted by Kelada F., "No abnormality could be discovered that might be related to such exposure," indicating the necessity of monitoring exposure levels in laboratory settings.
Additionally, JM Science Inc. emphasizes quality customer support and provides extensive resources, including how-to videos and application libraries, to assist its customers. This commitment improves the value proposition for laboratories and research institutions using dry chemical compounds in their work. Furthermore, there are 7 notifications provided by 150 of 4277 reports by companies with hazard statement code(s), underscoring the importance of safety and regulatory considerations associated with this specific salt.
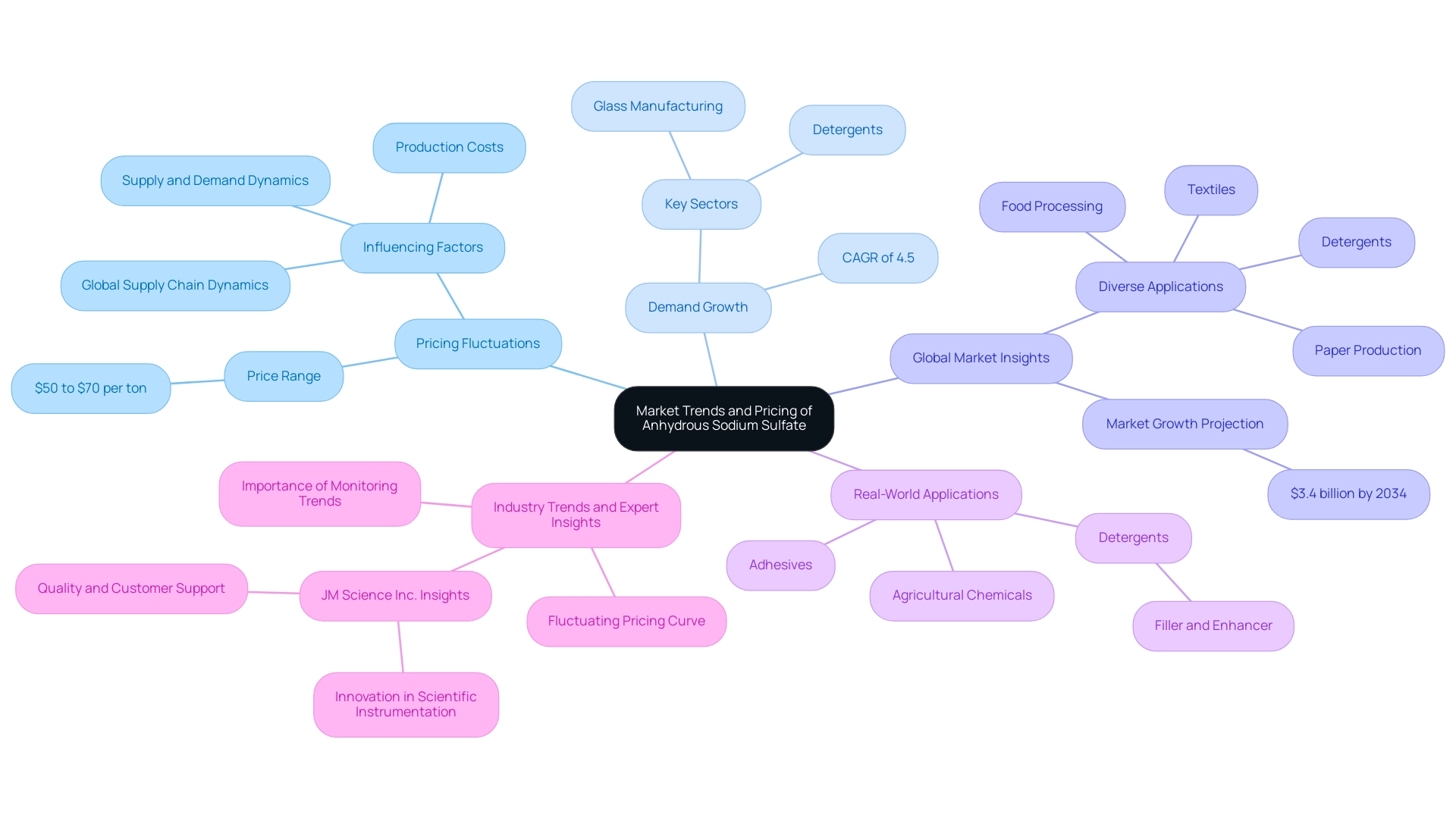
Market Trends and Pricing of Anhydrous Sodium Sulfate
The demand for dry natron has exhibited notable patterns in recent years, underscoring its critical role across multiple sectors.
- Pricing Fluctuations: The cost of anhydrous salt is influenced by the dynamics of supply and demand. Recent reports indicate a price range of $50 to $70 per ton within the U.S. sector. According to Procurement Resource, the global price trend for this chemical compound is anticipated to display a consistently varied curve, as evidenced in the fourth quarter of 2023. This variability emphasizes the need for stakeholders to remain vigilant about market conditions to optimize their procurement strategies.
The increasing demand for anhydrous sodium sulfate is particularly pronounced in the detergent and glass manufacturing sectors. This growth is projected to drive a compound annual growth rate (CAGR) of approximately 4.5% over the next decade, highlighting the compound's rising importance in enhancing product quality and efficiency.
- Global Market Insights: The global salt market is expected to reach around $3.4 billion by 2034. This growth is attributed to its diverse applications in industries such as detergents, textiles, paper production, and food processing, where it plays a vital role in improving product performance. A case study titled "Applications of Sodium Sulfate" illustrates its utilization across these sectors, emphasizing its significance in enhancing product quality and efficiency.
- Real-World Applications: In the detergent sector, a specific type of salt is utilized not only as a filler but also to enhance the solubility and effectiveness of cleaning agents. Its properties make it an indispensable component in formulating high-quality detergents that meet consumer demands for performance and sustainability. Furthermore, this salt is also employed in adhesives and agricultural chemicals, further demonstrating its versatility.
- Industry Trends and Expert Insights: Recent industry trends indicate a fluctuating pricing curve for this particular salt, reflecting variations influenced by factors such as production costs and global supply chain dynamics. Experts assert that understanding these trends is crucial for manufacturers and suppliers aiming to navigate the complexities of the industry effectively. JM Science Inc., with its dedication to quality, customer support, and innovation in scientific instrumentation, positions itself as a reliable partner for stakeholders in the salt sector, ensuring access to premium products and insights.
These insights into the anhydrous sodium sulfate market illustrate its significance and the necessity for ongoing monitoring of pricing and demand trends to facilitate strategic decision-making in procurement and production.
Environmental Impact and Regulatory Considerations of Anhydrous Sodium Sulfate
Anhydrous sodium compound is generally regarded as having a low environmental impact; however, several critical considerations must be addressed.
Regulatory Compliance: Laboratories and industries are mandated to comply with both local and international regulations regarding the use and disposal of this substance. Adherence to these regulations is essential for mitigating environmental risks and ensuring sustainable practices. For instance, facilities engaged in remedial actions as per EPA orders are exempt from certain fees but remain accountable for inspection and oversight costs, which can total up to $5,000. This underscores the importance of regulatory adherence and the financial implications of compliance, particularly concerning anhydrous sodium sulfate, which is non-toxic and biodegradable, making it a favorable option in various applications. Nevertheless, it is crucial for industries to implement strategies that minimize waste and promote recycling. By embracing sustainable methods, organizations can further reduce their ecological impact while utilizing effective salts. JM Science Inc. exemplifies this commitment by consistently refreshing its product range and nurturing robust connections with producers to address the evolving demands of the scientific community.
Impact on Aquatic Life: Although the chemical compound is not classified as hazardous, its discharge into aquatic environments must be handled with caution. Studies indicate that uncontrolled discharge can lead to alterations in water quality, potentially affecting aquatic ecosystems. Therefore, industries should establish protocols to monitor and control salt levels in wastewater to prevent adverse effects on marine life.
Environmental Statistics: Recent assessments reveal that while salts are widely utilized in industrial applications, their environmental impact remains relatively low compared to other chemical compounds. Nevertheless, ongoing observation and compliance with environmental guidelines are essential to uphold this status.
Real-World Compliance Examples: Various sectors have effectively implemented regulatory adherence measures for chemical compound usage, demonstrating best practices in waste management and environmental responsibility. JM Science Inc. is recognized for its commitment to quality and customer support, particularly through innovative products like electronic stethoscopes, which contribute to sustainable practices. These case studies illustrate the effectiveness of proactive approaches in minimizing environmental impact while ensuring operational efficiency. In summary, while the use of anhydrous sodium sulfate demonstrates a low environmental impact, it is imperative for industries to remain vigilant in their regulatory compliance and sustainability efforts to protect ecosystems and promote responsible usage.
Conclusion
Anhydrous sodium sulfate emerges as a critical compound with diverse applications across multiple industries, including detergents, glass production, textiles, pharmaceuticals, and laboratory settings. Its unique chemical properties, such as high solubility and hygroscopic nature, establish it as an essential component for enhancing product quality and efficiency. The various manufacturing processes, including the Mannheim process and evaporation of brine, underscore the compound's versatility and adaptability in meeting increasing market demands.
The market for anhydrous sodium sulfate is on an upward trajectory, propelled by growing demand in key sectors. Projections indicate a compound annual growth rate of approximately 4.5% over the next decade. Thus, stakeholders must remain informed about pricing fluctuations and market dynamics to optimize procurement strategies. The compound’s significant role in improving product performance across industries highlights its importance in the global chemical market.
Safety and regulatory compliance are paramount when handling anhydrous sodium sulfate. Implementing stringent safety protocols and adhering to environmental regulations are essential for mitigating potential risks associated with its use. As industries continue to evolve, maintaining sustainable practices will further enhance the compound's reputation as a low-impact chemical, ensuring its relevance in both current and future applications.
In conclusion, anhydrous sodium sulfate stands out as a versatile and indispensable compound in industrial chemistry. Its contributions to various sectors, coupled with the need for responsible handling and compliance, reinforce its significance in promoting efficiency and sustainability in modern manufacturing practices. As the landscape of industrial applications continues to evolve, understanding the dynamics surrounding anhydrous sodium sulfate will be crucial for stakeholders aiming to leverage its full potential.




