Overview
This article delves into the benefits, risks, and regulatory standards surrounding phosphates in drinking water. It underscores their vital role in water treatment while acknowledging the potential health and environmental risks associated with excessive levels.
Phosphates significantly enhance water quality through effective corrosion control and microbial management. However, the necessity for diligent monitoring and regulation is paramount to prevent eutrophication and the health issues that can arise from it.
By addressing these aspects, the article highlights the importance of balancing the advantages of phosphates with the need for responsible management.
Introduction
In the intricate balance of our ecosystems, phosphates emerge as pivotal players, significantly influencing water quality and aquatic health. These essential chemical compounds sustain life; however, their excessive presence can lead to substantial challenges, including eutrophication and health risks for vulnerable populations. The journey through the multifaceted role of phosphates in drinking water reveals a complex narrative—one that encompasses their benefits in water treatment, the risks associated with overexposure, and the regulatory frameworks designed to safeguard public health.
As communities grapple with the implications of nutrient pollution, understanding the dynamics of phosphates becomes crucial for ensuring the sustainability of water resources and the well-being of those who rely on them.
Define Phosphates and Their Role in Drinking Water
Phosphates are chemical compounds characterized by the ion (PO4^3-), serving as essential nutrients for both plants and animals. In drinking fluids, phosphates primarily originate from agricultural runoff, wastewater discharge, and various industrial processes. Their presence is vital for maintaining quality in aquatic environments, as they stimulate the growth of aquatic plants and algae. While this growth can be advantageous in controlled settings, it poses significant risks in natural aquatic systems, potentially leading to eutrophication—a process that diminishes oxygen levels and disrupts marine ecosystems.
Recent assessments reveal that 1% of rivers exhibit average nitrate concentrations exceeding 37.5 mg/l NO3, underscoring the ongoing challenges posed by nutrient contamination. Furthermore, case studies illustrate that facilities can achieve less stringent phosphorus restrictions through site-specific criteria, provided they meet local quality objectives despite elevated phosphorus levels. This flexibility allows for compliance while acknowledging local quality achievements. The role of orthophosphate in managing lead and copper release in phosphate systems is also significant, highlighting the practical implications of effective management. Environmental scientists emphasize that regulating phosphorus levels is crucial to mitigate risks associated with agricultural runoff and other sources, thereby securing the sustainability of aquatic resources and the health of marine ecosystems. As Matt Claucherty notes, understanding the economic implications linked to nutrient management is essential for facilities aiming to optimize their operations. Additionally, phosphorus operational assessment and enhancement report worksheets can serve as vital tools for efficiently managing phosphate concentrations in facilities, while understanding the concentration and sources of phosphates in beverages is essential for assessing their effects on human health and ecological systems.
Explore the Benefits of Phosphates in Drinking Water
Phosphates drink quality is pivotal when maintained at appropriate levels. They are vital for sustaining nutrient equilibrium in aquatic ecosystems, fostering the growth of beneficial microorganisms that improve overall quality. In treatment plants, phosphorus compounds play a crucial role in preventing corrosion within pipes, significantly reducing the leaching of toxic metals such as lead and copper into drinking water. This corrosion control is essential for protecting public health.
Moreover, these substances boost the efficiency of various liquid treatment methods, including coagulation and flocculation. Such processes are critical for removing impurities and improving the overall clarity of the liquid. Statistical analyses reveal that treatments with phosphorus can lead to substantial changes in microbial community structures, with the average relative abundance of transport and metabolism of amino acids being 9.3% in control and 7.2% in treatment. This dynamic illustrates the beneficial impact of nutrients on microbial ecology within drinking supply distribution systems, particularly supporting fungal populations while maintaining bacterial stability.
Real-world examples underscore the advantages of these compounds in preventing corrosion. Case studies, such as the 'Statistical Analysis of Microbial Communities,' demonstrate that the strategic application of specific compounds not only protects infrastructure but also enhances the overall efficiency of treatment operations. Experts in liquid purification emphasize that the use of phosphates drink is essential for improving treatment processes and ensuring the delivery of safe, high-quality drinking water to communities. As noted in the study, "We thank Yorkshire Water and especially Jessica Lindquist and Jenny Banks for guidance on dosing and for supplying equipment to measure physico-chemical parameters." Furthermore, phosphorus compounds are acknowledged for their role in eliminating biofilm and enhancing disinfection efficiency in aquatic systems, further highlighting their critical function in treatment processes.
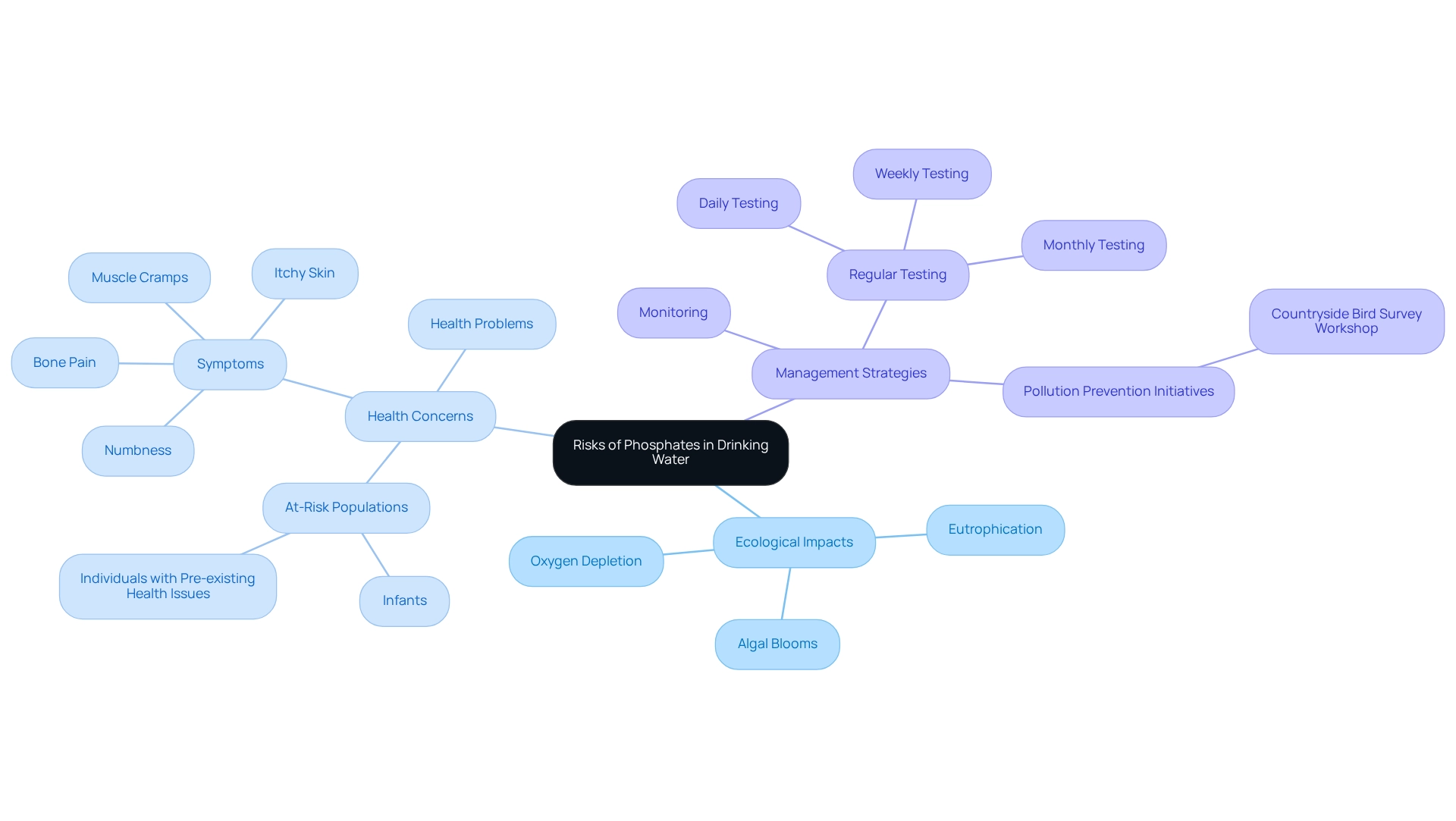
Assess the Risks of Phosphates in Drinking Water
While these compounds serve vital roles in moderation, their overabundance in phosphates drink can lead to significant repercussions. Increased levels of phosphorus contribute to eutrophication, a harmful process characterized by nutrient excess that triggers uncontrolled algal blooms. These blooms diminish oxygen concentrations in water bodies, resulting in considerable damage to aquatic ecosystems and biodiversity.
Furthermore, studies indicate a connection between elevated phosphorus concentrations and various health problems, particularly among at-risk populations such as infants and individuals with pre-existing health issues. Signs of excessive phosphorus consumption—including muscle cramps, numbness, bone pain, and itchy skin—underscore the urgent need for vigilant monitoring of phosphorus levels.
Regulatory bodies assert that managing phosphorus in phosphates drink sources is crucial for safeguarding human health. Regular testing for total phosphorus—whether conducted daily, weekly, or monthly—should align with facility requirements and regulations to mitigate these risks.
Effective strategies to combat nutrient pollution are essential for protecting ecosystems and ensuring the well-being and security of communities reliant on these resources. Initiatives such as the Countryside Bird Survey Workshop exemplify efforts to enhance biodiversity monitoring and conservation, which are vital in addressing the adverse effects of pollution from harmful substances.
Understand Regulatory Standards for Phosphate Levels
Regulatory standards for phosphates drink and phosphorous compounds in drinking water are crucial for safeguarding public health, typically established by environmental protection agencies. In the United States, the Environmental Protection Agency (EPA) sets maximum contamination thresholds (MCLs) for various substances, including specific compounds, based on extensive scientific research and risk evaluations.
While not all substances have universally established MCLs, the EPA emphasizes the importance of monitoring concentrations to prevent potential health risks associated with excessive exposure. Compliance with these regulations is vital for water treatment facilities, which are required to engage in regular monitoring and reporting to meet established standards.
Noncompliance with treatment technique requirements constitutes a regulatory violation, highlighting the necessity of stringent oversight. For instance, the EPA's Interim Health Advisory for perchlorate serves as a clear example of how regulatory standards inform cleanup decisions and protect vulnerable populations.
Case studies demonstrate that facilities adhering to these standards significantly contribute to community health, underscoring the importance of maintaining nutrient levels within regulatory limits. Furthermore, if a sample result falls below the Practical Quantitation Limit (PQL), zero is recorded for that analyte in compliance calculations, emphasizing the critical role of accurate measurement in adhering to phosphates drink regulations.
Conclusion
Phosphates serve a dual role in drinking water, acting as both essential nutrients and potential hazards. Their presence is vital for maintaining water quality and supporting aquatic ecosystems; however, excessive phosphate levels can lead to significant environmental challenges, such as eutrophication and adverse health effects for vulnerable populations. Striking a balance between harnessing the benefits of phosphates in water treatment and managing their associated risks is crucial for sustainable water resource management.
Effective management strategies and regulatory frameworks are imperative to ensure phosphate levels remain within safe limits. Regulatory bodies like the EPA are instrumental in establishing standards and monitoring compliance to safeguard public health. Regular testing and adherence to these guidelines not only protect drinking water quality but also contribute to the overall health of aquatic ecosystems.
As communities face the complexities of nutrient pollution, a comprehensive understanding of phosphates and their implications becomes increasingly important. By prioritizing responsible phosphate management and adhering to regulatory standards, it is possible to leverage the benefits of phosphates while minimizing their risks. This approach ultimately ensures the sustainability of water resources for both current and future generations.




