Overview
This article delves into the mastery of ion suppression techniques within pharmaceutical analysis, underscoring their vital importance in achieving accurate quantification and maintaining data integrity in mass spectrometry. It presents a range of strategies designed to mitigate ion suppression, including:
- Optimization of sample preparation
- Use of specific additives
These strategies are essential for enhancing the reliability of analytical results and ensuring compliance with regulatory standards. By understanding and implementing these techniques, professionals can significantly improve the quality of their analytical outcomes.
Introduction
Ion suppression represents a formidable challenge in pharmaceutical analysis, where the accuracy of results is paramount for ensuring patient safety and meeting regulatory standards. The presence of certain ions can significantly diminish the ionization efficiency of target analytes, making it crucial for analysts to comprehend this phenomenon in their quest to enhance data integrity.
What strategies can be implemented to mitigate the widespread effects of ion suppression? Moreover, how can analysts assure that their methods produce reliable results despite this persistent issue? Addressing these questions is essential for advancing the field and safeguarding the integrity of pharmaceutical analyses.
Define Ion Suppression and Its Importance in Pharmaceutical Analysis
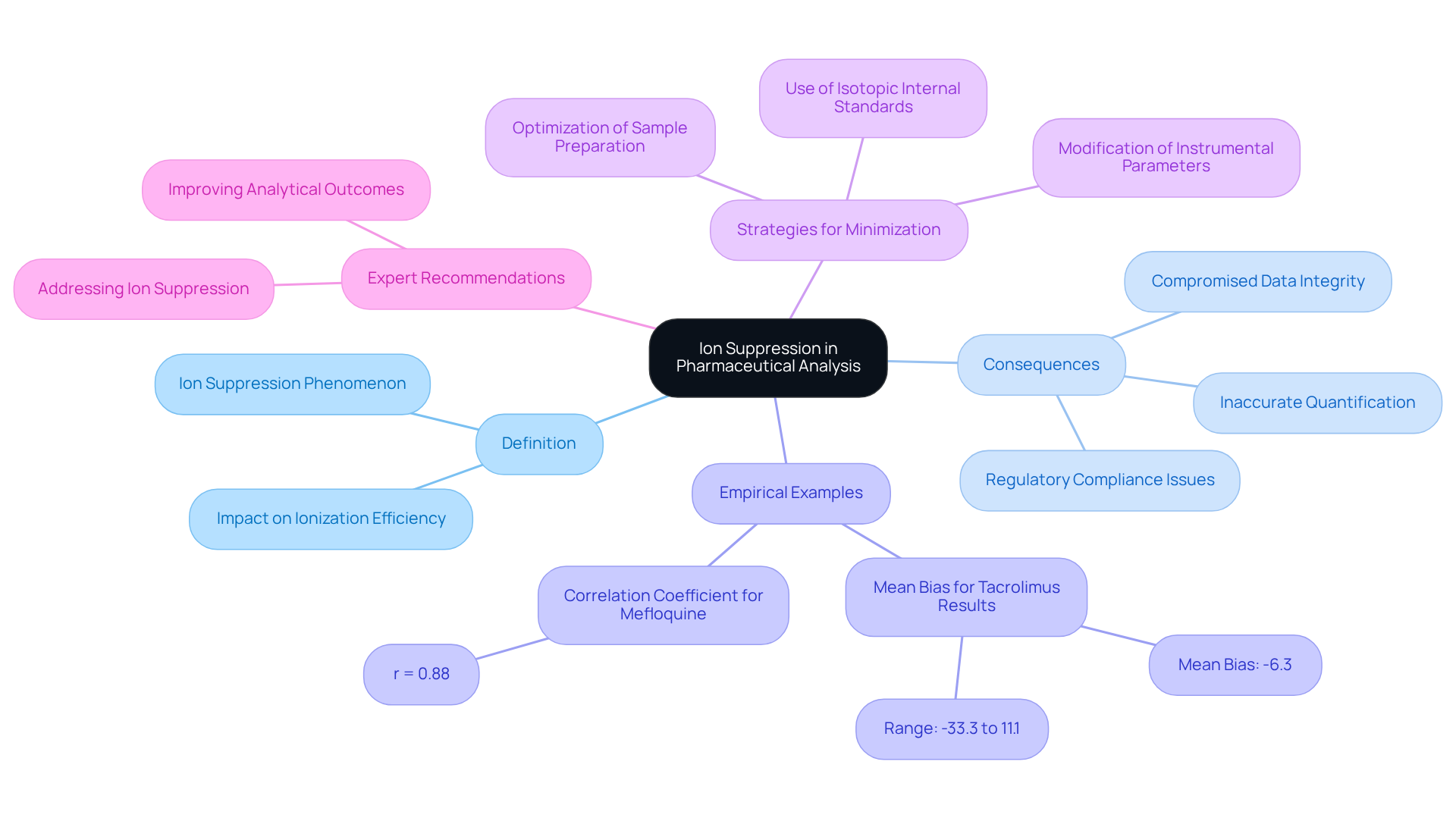
Ion suppression is a critical phenomenon in mass spectrometry, where the presence of specific ions within a sample matrix diminishes the ionization efficiency of target analytes. This reduction can lead to inaccurate quantification and compromised , particularly in pharmaceutical analysis, where precision is paramount.
Recent studies underscore that ion suppression can significantly impact detection limits and the overall performance of analytical methods, emphasizing its importance in method validation and regulatory compliance. For instance, a mean bias of -6.3% for tacrolimus results illustrates how ion interference can distort outcomes and lead to erroneous conclusions. Additionally, the correlation coefficient (r = 0.88) between salivary and plasma mefloquine concentrations further demonstrates the detrimental effect of ion reduction on data integrity.
By understanding the implications of ion reduction, analysts can design more robust experiments and accurately interpret results, ultimately enhancing the reliability of pharmaceutical products. Case studies targeting strategies for minimizing ion interference reveal that employing specific techniques can improve analytical outcomes, highlighting the necessity for thorough evaluation during method development.
As industry leaders assert, addressing ion suppression is vital for ensuring the accuracy and reliability of mass spectrometric analyses in the pharmaceutical sector. Expert quotes emphasize that utilizing isotopic internal standards is essential for achieving reliable quantification and high precision, further reinforcing the critical nature of this phenomenon.
Explore Mechanisms of Ion Suppression in Chromatography
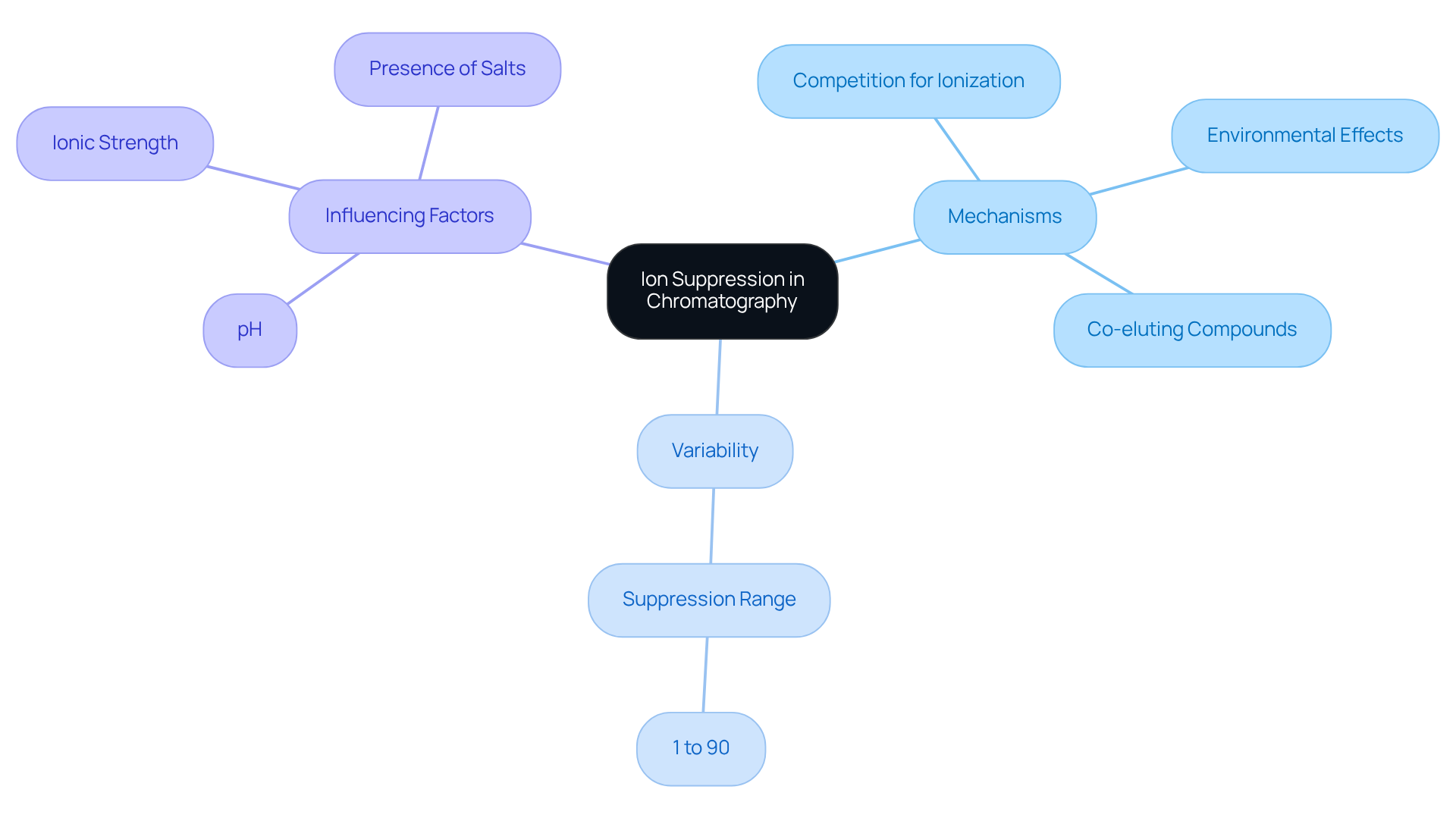
Ion suppression in liquid chromatography-mass spectrometry (LC-MS) is a critical phenomenon that arises from various mechanisms, including:
- Competition for ionization in the gas phase
- Environmental effects from the sample
- The presence of co-eluting compounds
This arrangement can introduce various ions that compete for ionization alongside target analytes, resulting in ion suppression and diminished signal intensity. Recent research underscores that ion suppression can lead to measurement errors, with the levels of suppression showing considerable variability—ranging from 1% to over 90%—depending on sample composition and analyte characteristics. Notably, ion suppression occurs for nearly all analytes and in every environment examined for herbicides in surface waters, highlighting the pervasive nature of this issue.
Factors such as:
- pH
- Ionic strength
- The presence of salts within the sample matrix
play a crucial role in influencing ion suppression efficiency. Furthermore, the efficacy of sample preparation techniques varies in their ability to eliminate interferences, with liquid-liquid extraction (LLE) generally yielding cleaner extracts compared to solid-phase extraction (SPE). Understanding these mechanisms is essential for the development of that effectively mitigate ion suppression, thereby enhancing the reliability and precision of LC-MS analyses. As Dietrich A. Volmer aptly states, 'the limited understanding of the origin and mechanism of ion reduction makes this problem challenging to resolve in many instances.' Additionally, the FDA's Guidance for Industry on Bioanalytical Method Validation emphasizes the importance of addressing ion suppression to ensure the integrity of analytical results.
Implement Detection and Evaluation Techniques for Ion Suppression
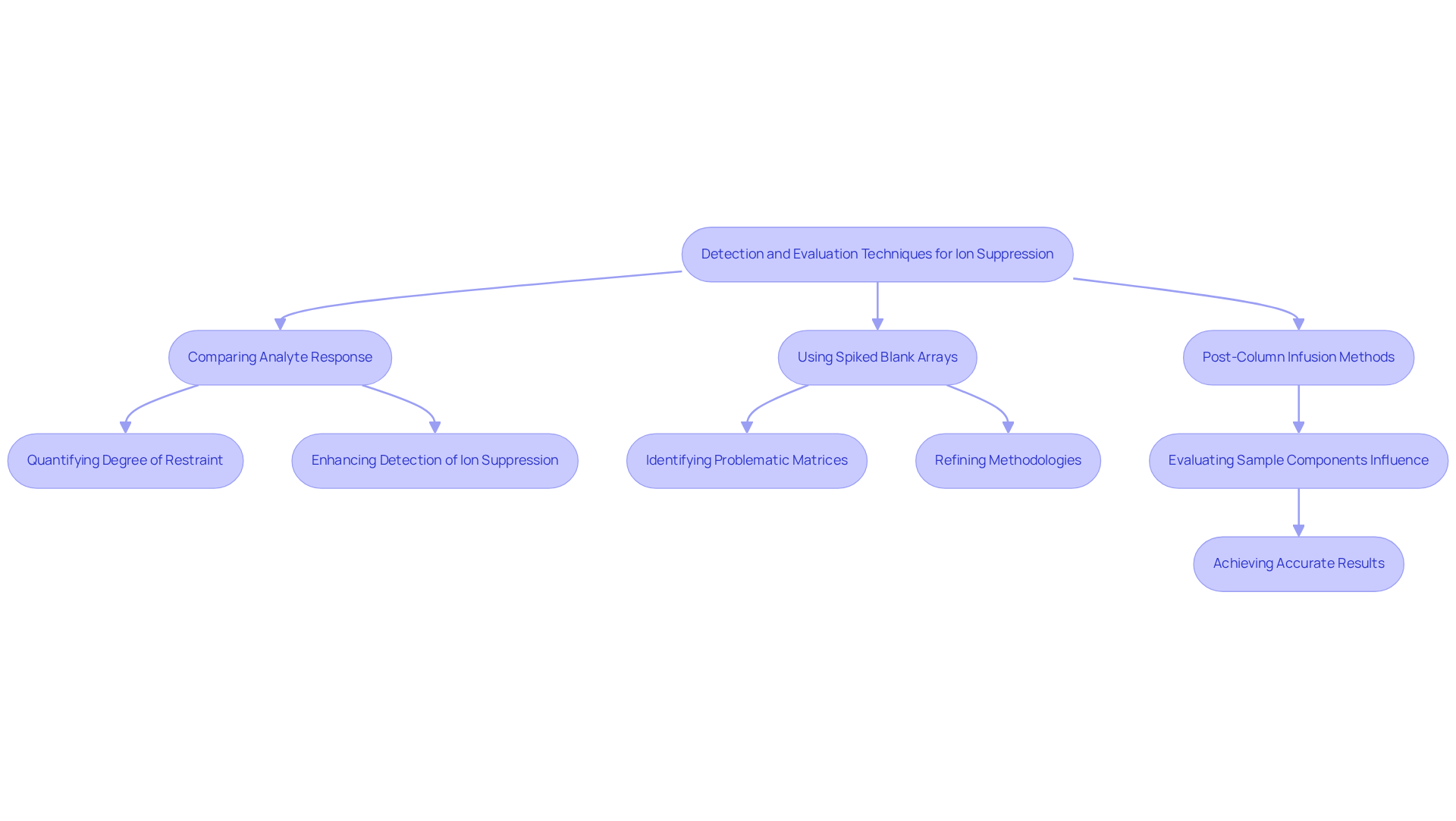
To effectively , a variety of techniques can be employed. A prevalent approach involves comparing the analyte's response in a spiked blank medium with that in a standard solution. This comparison quantifies the degree of restraint, providing critical insights into the analytical process.
Statistics indicate that utilizing spiked blank arrays significantly enhances the detection of ion suppression, with research revealing that 76-83% of features show improved responses under these conditions. Furthermore, post-column infusion methods serve as a robust means to evaluate how sample components influence ionization. By systematically analyzing these effects, analysts can identify problematic matrices and refine their methodologies accordingly.
This proactive adjustment is essential for achieving more accurate and reliable results in pharmaceutical analysis. It is also crucial to recognize that ion suppression can lead to false negatives or positives in analytical findings, underscoring the importance of comprehensive assessments in quality assurance.
Apply Strategies to Mitigate Ion Suppression Effects
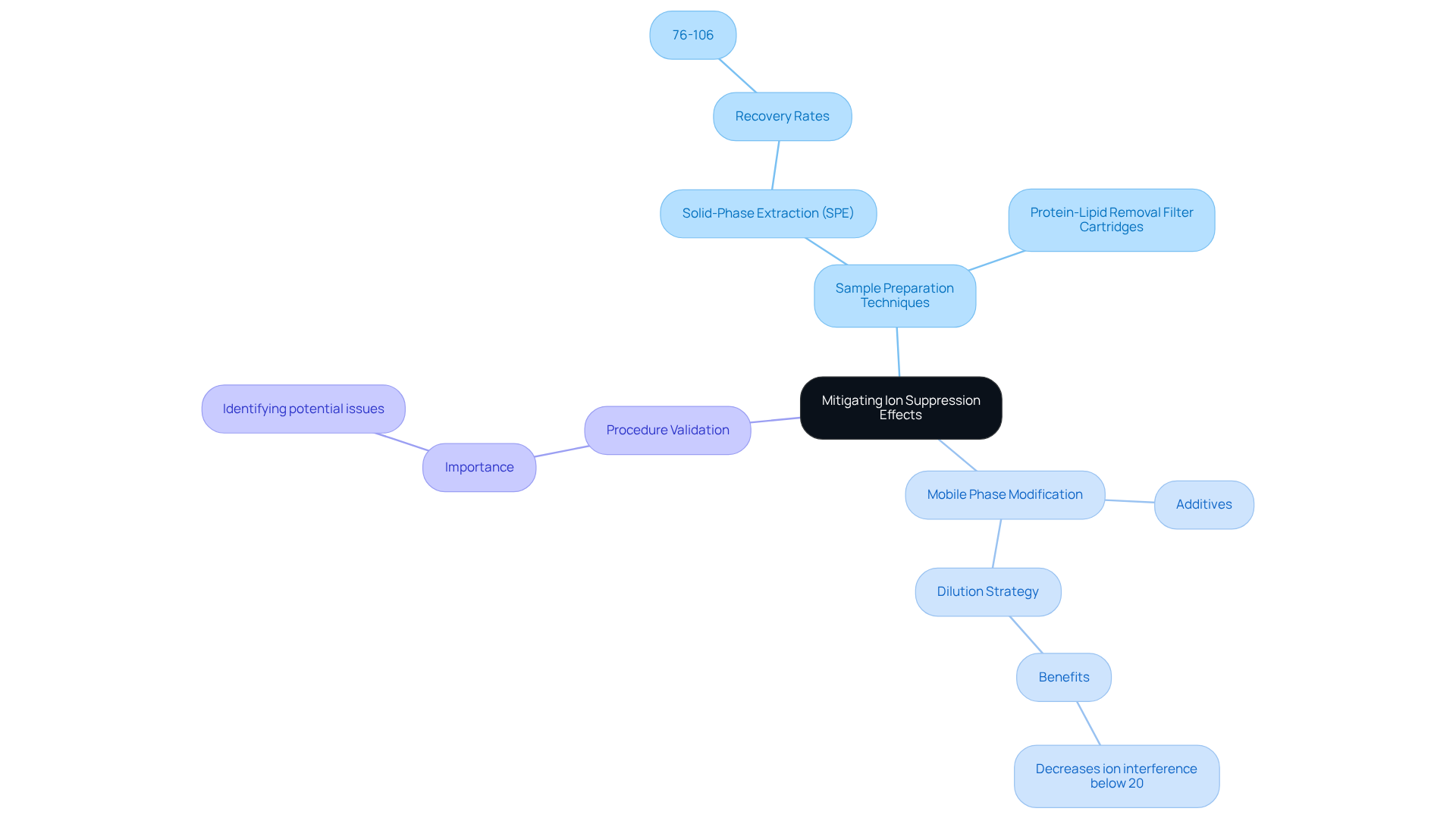
To effectively mitigate the effects of ion suppression in pharmaceutical analysis, several strategies can be employed. Optimizing sample preparation techniques, particularly solid-phase extraction (SPE), has proven to be highly effective. Research indicates that SPE can achieve recovery rates ranging from 76% to 106% for mycotoxins in rice, showcasing its substantial capability to reduce interference effects. Additionally, the utilization of protein-lipid removal filter cartridges, such as Captiva ND Lipids, has emerged as one of the premier options for minimizing matrix effects in LC-MS applications.
Modifying the mobile phase composition by incorporating specific additives can further enhance ionization efficiency. For instance, the dilution strategy has demonstrated effectiveness; it is efficient, simple to execute, and quick, particularly when it does not significantly compromise the required sensitivity of the technique. This approach has proven effective in decreasing ion interference to below 20% when initial interference is substantial. Moreover, implementing stringent procedure validation protocols that evaluate ion suppression is essential. This proactive approach facilitates the within the analytical process.
Expert insights underscore the critical nature of these strategies. As noted, "When the available calibration strategy does not completely compensate for matrix effects, or the method lacks in sensitivity, it is crucial to take measures to reduce or eliminate matrix effects." By applying these strategies, analysts can significantly enhance the reliability of their results and ensure compliance with regulatory standards, ultimately leading to more accurate and reproducible analytical outcomes.
Conclusion
Understanding and addressing ion suppression is essential for achieving accurate and reliable results in pharmaceutical analysis. This phenomenon can significantly diminish ionization efficiency and compromise data integrity, underscoring the critical nature of method validation and regulatory compliance in the sector. By recognizing the implications of ion suppression, analysts can implement strategies that enhance the robustness of their experiments, ultimately improving the overall reliability of pharmaceutical products.
The article delves into the mechanisms behind ion suppression, including factors such as competition for ionization and the influence of sample composition. It emphasizes the importance of evaluating ion suppression through various detection techniques and the application of effective mitigation strategies, such as optimizing sample preparation and modifying mobile phase compositions. These insights underscore the necessity for thorough assessments in quality assurance and the proactive identification of potential issues within analytical processes.
In conclusion, the significance of ion suppression in pharmaceutical analysis cannot be overstated. As the industry continues to evolve, the adoption of best practices and innovative techniques will be paramount in ensuring the accuracy and reliability of analytical outcomes. By prioritizing the understanding and management of ion suppression, analysts can contribute to the advancement of pharmaceutical testing, ultimately leading to safer and more effective therapeutic products for consumers.




