Overview
The article underscores the critical importance of the KF value in pharmaceutical laboratories, particularly within the realm of Karl Fischer titration for moisture analysis.
Mastery of the KF value is not merely beneficial; it is essential for achieving accurate moisture determination.
This value quantifies the impact of solutes on the freezing point of solvents, thereby influencing the reliability of analytical results in high-stakes environments like pharmaceuticals.
Understanding this relationship is vital for professionals in the field who seek to uphold the integrity of their analytical processes.
Introduction
The interplay between solutes and solvents constitutes a fundamental concept in chemistry, particularly in understanding the properties of solutions. One such property, freezing point depression, transcends mere academic curiosity; it plays a critical role in pharmaceutical applications, where precise measurements directly influence product quality and safety. This article explores the significance of the KF value, detailing its impact on moisture analysis in laboratory settings and the challenges researchers encounter in its application.
What key insights can enhance the reliability of moisture determination? Furthermore, how can laboratories adeptly navigate the complexities associated with the KF value to ensure accurate results?
Explore Freezing Point Depression: Theoretical Foundations
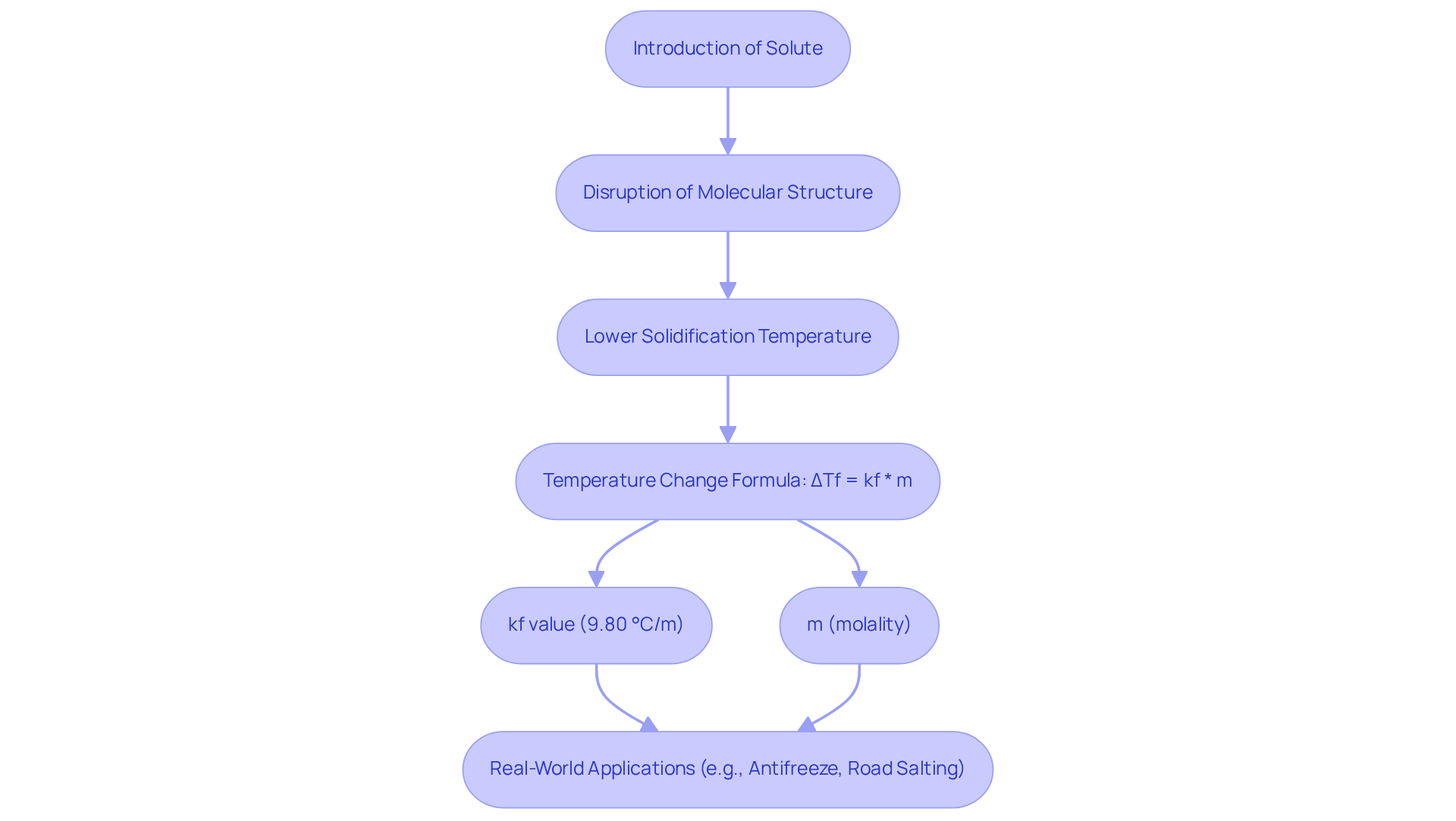
The lowering of the solidification temperature represents a critical colligative property that emerges when a solute is introduced to a liquid, resulting in a solidification temperature that falls below that of the pure liquid. This phenomenon occurs due to the disruption of the liquid's molecular structure, which necessitates a lower temperature to attain the solid state.
Importantly, the extent of this temperature reduction is directly proportional to the molality of the solute, as expressed in the equation:
ΔTf = kf value * m.
In this equation:
- ΔTf signifies the change in temperature,
- kf value represents the molal lowering temperature constant specific to the liquid (9.80 °C/m),
- m denotes the molality of the solution.
Understanding this relationship is vital for accurately determining water content in samples through , as it significantly influences the calculations involved in the process.
Recent studies have demonstrated that the inclusion of solutes not only lowers the temperature at which the liquid solidifies but also alters the chemical potential of the solvent, a factor that is crucial for various laboratory applications.
For example, in analytical chemistry, a comprehensive understanding of colligative properties enables precise measurements, thereby enhancing the reliability of experimental outcomes.
Real-world applications, such as the use of antifreeze in vehicles, further underscore the practical implications of lowered temperature thresholds, highlighting their importance in both laboratory and industrial settings.
Moreover, the highest drop in temperature due to road salting is approximately −18 °C, which emphasizes its significance in real-world scenarios.
As Tyreke Davis aptly noted, "When a liquid is not pure and contains particles dissolved within it, there are constituents that occupy space in the mixture," underscoring the importance of comprehending these interactions.
Define KF Value: Importance in Freezing Point Calculations
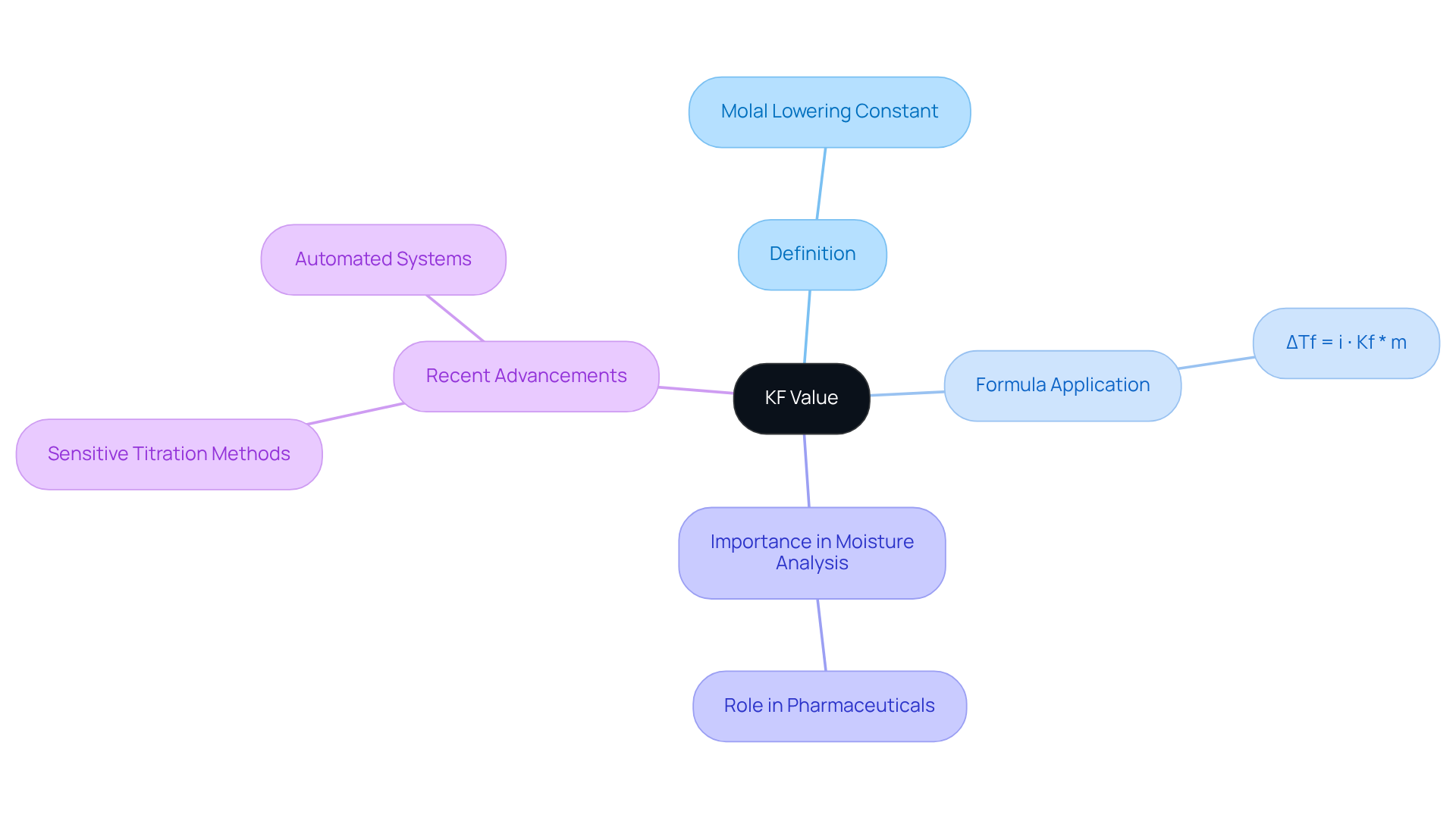
The KF value, which is defined as the molal lowering constant, is a critical solution-specific parameter that quantifies how much a solute can lower the temperature at which a liquid solidifies. For example, the kf value for water is approximately 1.86 °C kg/mol. This constant is pivotal in , allowing analysts to accurately determine the water content in various samples.
By applying the formula ΔTf = i ⋅ Kf * m—where ΔTf signifies the observed freezing point depression, i is the van't Hoff factor, and m is the molality of the solution—professionals can ascertain the precise amount of water present.
As Jules Bruno articulates, "The presence of solute particles means that the solvent molecules must lose more kinetic energy than they would have to if the solute were not present, in order to form the orderly crystal structure of ice."
Mastery of the kf value is indispensable for achieving accurate and reliable results in moisture analysis, particularly within the pharmaceutical sector, where precision is essential for product quality and compliance.
Recent advancements in KF applications, including the development of more sensitive titration methods and the integration of automated systems, have significantly enhanced its utility. These innovations facilitate more efficient moisture determination in complex matrices, thereby bolstering rigorous quality control processes in laboratories.
Address Challenges: Navigating KF Value Applications in the Lab
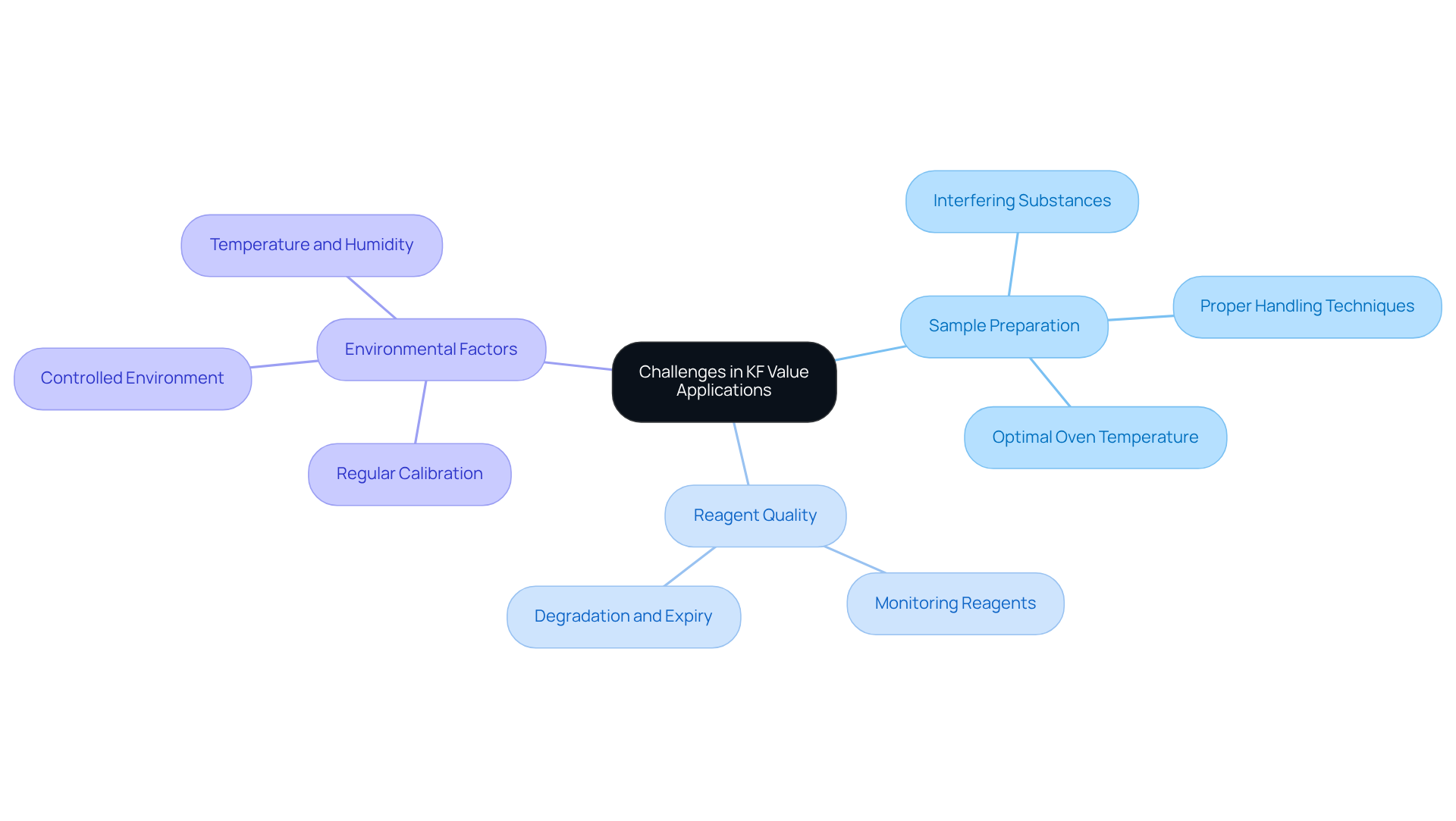
Navigating the application of the kf value in the laboratory presents several challenges that demand attention. Issues such as sample preparation, reagent quality, and environmental factors can significantly impact the accuracy of moisture analysis. One prevalent concern is the presence of interfering substances in samples, which can lead to inaccurate readings. To mitigate this risk, it is crucial to ensure that samples are and that any potential contaminants are thoroughly removed.
Furthermore, the quality of the KF reagents must be closely monitored. Degraded or expired reagents can severely affect the accuracy of titration results, undermining the reliability of the analysis. Environmental factors, including temperature and humidity, also play a critical role in the performance of the KF titration process, particularly affecting the kf value. Maintaining a controlled laboratory environment and regularly calibrating equipment are essential practices that can help address these challenges.
By implementing these strategies, laboratories can ensure reliable and precise moisture analysis. The importance of high-quality scientific instruments cannot be overstated, as they are fundamental to achieving accurate results in laboratory settings.
Conclusion
Mastering the KF value is essential for pharmaceutical laboratories, as it plays a pivotal role in accurately determining the water content in samples. Understanding the theoretical foundations of freezing point depression and the significance of the KF value not only enhances analytical precision but also ensures compliance with industry standards.
Critical insights into the relationship between solute concentration and freezing point depression, the importance of reagent quality, and the challenges laboratories face in achieving accurate moisture analysis have been discussed. By employing rigorous sample preparation methods and maintaining a controlled environment, laboratories can effectively navigate these challenges, ensuring reliable results in moisture determination.
In conclusion, mastery of the KF value and its applications is vital for maintaining product quality in the pharmaceutical sector. As advancements in titration methods continue to evolve, embracing best practices for measuring the KF value will empower laboratories to enhance their quality control processes. Prioritizing accuracy in moisture analysis not only supports compliance but also contributes to the overall integrity of pharmaceutical products, ultimately benefiting both manufacturers and consumers.




