Overview
This article delves into the mastery of the titration process, underscoring the critical role of a titrant in accurately determining the concentration of an analyte. It meticulously outlines the essential materials and provides a step-by-step procedure, addressing common troubleshooting issues along the way. By emphasizing the significance of precise techniques and proper equipment calibration, the article illustrates how these practices can significantly enhance the reliability and precision of analytical results across various laboratory environments.
Introduction
Titration stands as a cornerstone of analytical chemistry, offering a precise method for determining the concentration of unknown substances through systematic chemical reactions. This guide presents an opportunity for readers to master the intricacies of titration, encompassing essential terminology and empowering them to execute the procedure with confidence. However, the journey toward accurate results is not without its challenges—how can one navigate common pitfalls and ensure reliable outcomes in a laboratory setting?
Understand the Basics of Titration
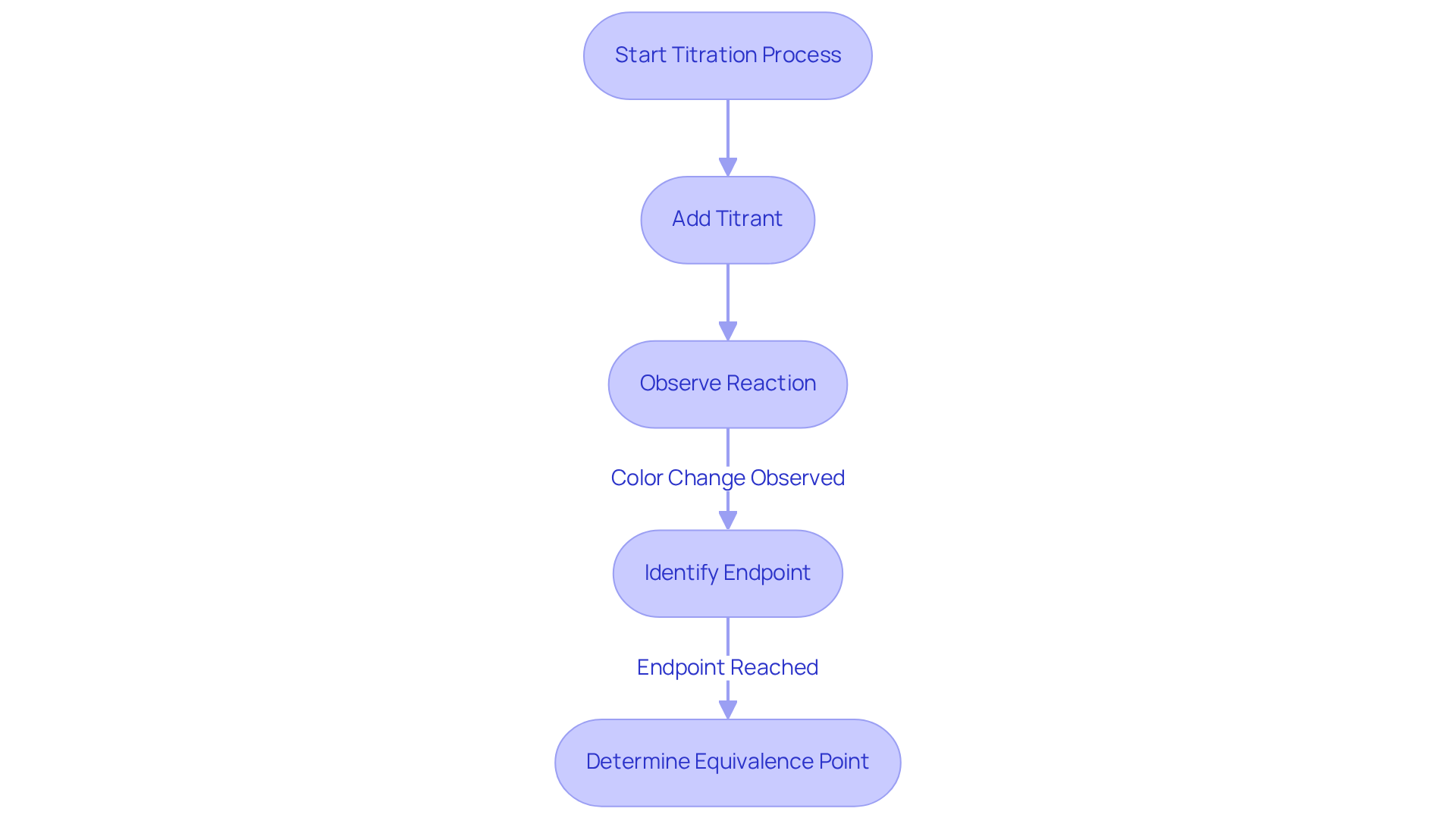
Titration stands as a quantitative analytical method essential for determining the concentration of an unknown substance, known as the analyte. This process involves systematically adding a liquid of known concentration, referred to as the reagent, until the reaction reaches completion. The endpoint of the titration is typically indicated by a noticeable color change or a measurable alteration in a characteristic of the liquid, such as pH. Mastery of the stoichiometry involved in the reaction is paramount, enabling precise calculations of the analyte's concentration based on the volume of the titrant and the amount of titrate consumed. Key terms to understand include:
- Titrant: The solution with a known concentration used to react with the analyte, often provided by premium titrators from JM Science.
- Analyte: The solution whose concentration is unknown and is being measured.
- Endpoint: The stage at which the reaction is deemed complete, often marked by a noticeable color change.
The equivalence point is the moment when the quantity of titrant added is stoichiometrically equivalent to the amount of titrate present.
In 2025, approximately 75% of laboratories reported employing volumetric analysis techniques, underscoring their significance in analytical chemistry. The importance of stoichiometry in this process cannot be overstated, as it ensures accurate results and reliable data interpretation. find extensive applications across various fields, including pharmaceuticals, environmental testing, and food quality control, highlighting their versatility and relevance in real-world scenarios. JM Science Inc. offers a comprehensive range of products, including Karl Fischer reagents, various HPLC columns, and accessories, which enhance the accuracy and efficiency of analytical processes in pharmaceutical labs. As noted by chemists, the accuracy of titrant and titrate is essential for obtaining precise quantitative analysis, making this technique an indispensable tool in the laboratory.
Gather Required Materials and Equipment
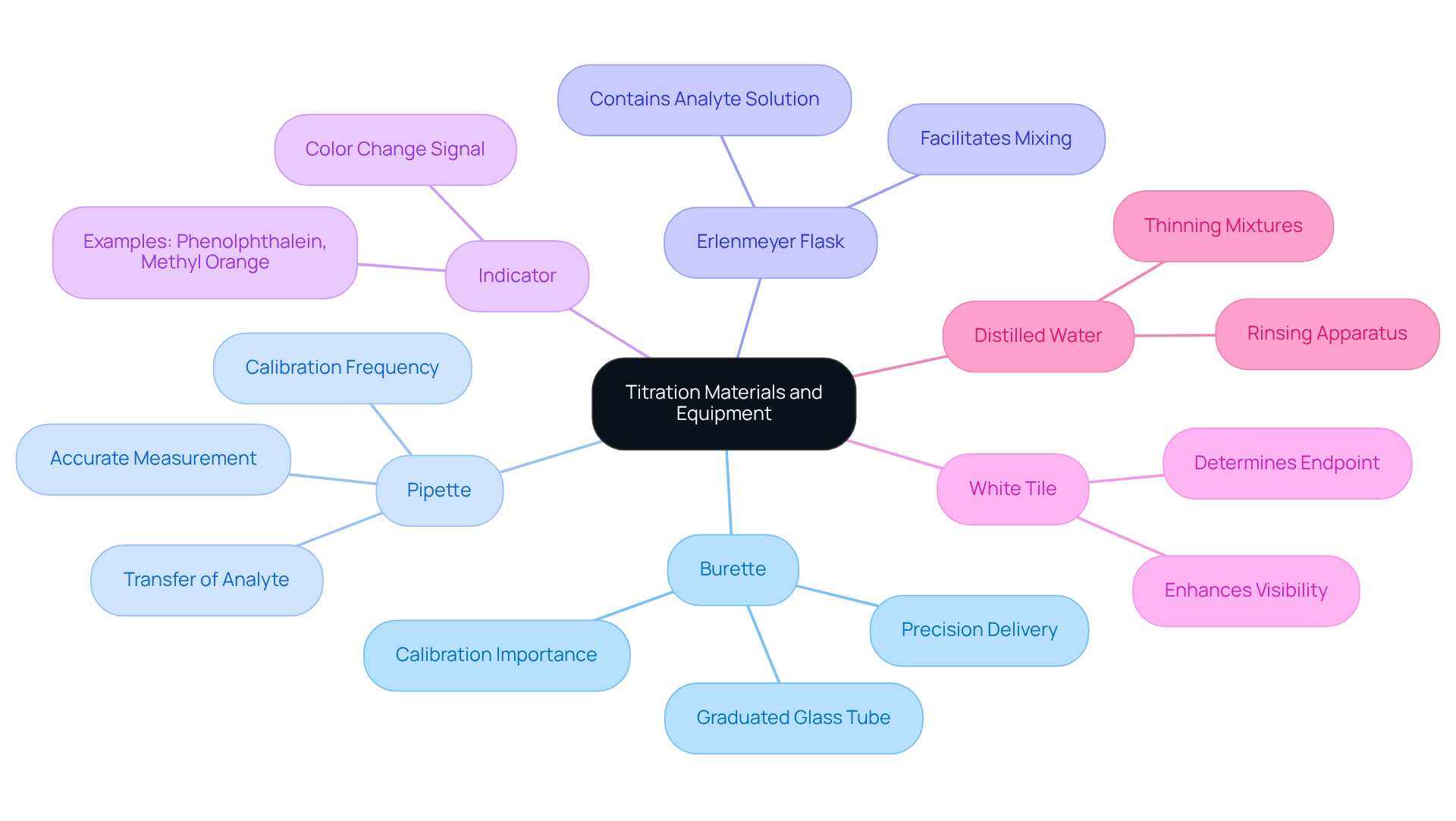
To successfully perform a titration, it is essential to gather the following materials and equipment:
- Burette: A graduated glass tube equipped with a tap at one end, crucial for delivering the titrant with precision.
- Pipette: This instrument is used for accurately measuring and transferring a specific volume of the analyte, ensuring consistency in your measurements.
- Erlenmeyer Flask: The flask contains the analyte solution during the measuring process, allowing for easy mixing and observation.
- Indicator: A substance that signals the conclusion of the titration through a color change, such as phenolphthalein or methyl orange, providing visual confirmation of the reaction's completion.
- White Tile: Placing the flask on a white tile enhances the visibility of color changes, making it easier to determine the endpoint.
- Distilled Water: This is crucial for rinsing apparatus and thinning mixtures as required, guaranteeing that no impurities influence your outcomes.
The solution of known concentration, known as the titrant, must be prepared and standardized prior to use to ensure precise titrate measurement outcomes.
It is imperative that all equipment is thoroughly cleaned and calibrated to minimize contamination and measurement errors. Regular calibration of the burette, ideally every 6-12 months, is recommended to maintain accuracy in liquid delivery. In pharmaceutical laboratories, where precision is paramount, employing calibrated equipment enhances the reliability of results and aligns with industry standards for quality control. Real-world examples illustrate that careful preparation of measurement setups can greatly influence the results of analyses, emphasizing the importance of adhering to best practices in laboratory environments.
Follow the Step-by-Step Titration Procedure
To perform a titration effectively using JM Science Inc.'s premium titrators, it is essential to follow these steps meticulously:
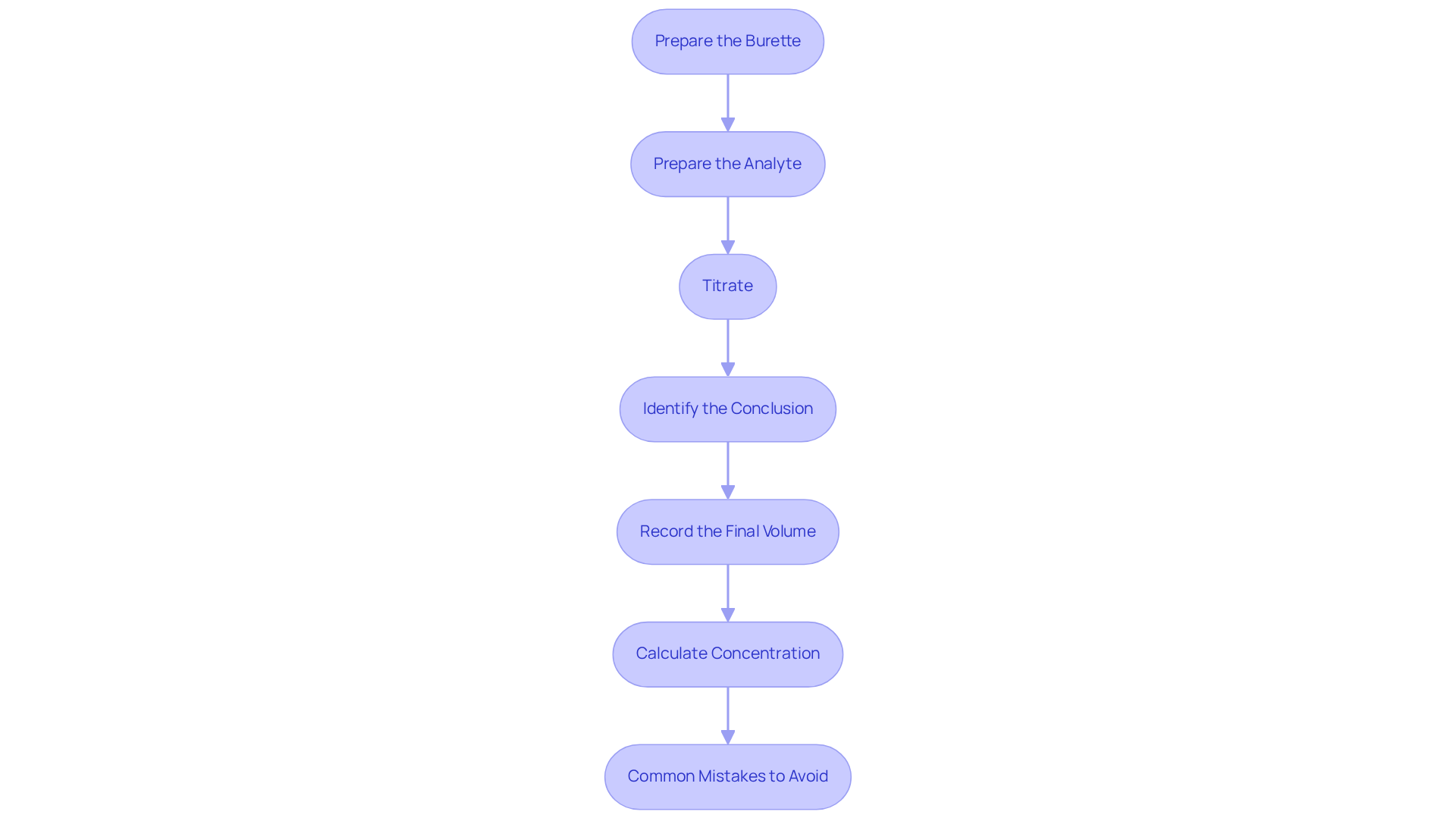
- Prepare the Burette: Begin by rinsing the burette thoroughly with distilled water, followed by the reagent to prevent contamination. Fill the burette with the titrant and then titrate by accurately recording the initial volume.
- Prepare the Analyte: Measure a specific volume of the analyte solution using a pipette and transfer it to an Erlenmeyer flask. Incorporate a few drops of the chosen indicator to the analyte to facilitate visual identification of the conclusion.
- Titrate: Position the Erlenmeyer flask on a white tile to enhance visibility. Gradually add the titrant from the burette to the analyte while continuously swirling the flask to titrate the solution. Watch for a color change that signifies the conclusion.
- Identify the Conclusion: As you near the conclusion, add the reagent drop by drop to avoid overshooting. The conclusion is confirmed when a lasting color change is observed. Employing appropriate indicators or a pH meter can further enhance accuracy in this critical step.
- Record the Final Volume: Upon reaching the endpoint, document the final volume of the solution in the burette. Calculate the volume of the solution used by subtracting the initial volume from the final volume recorded.
- Calculate Concentration: Apply the formula to determine the concentration of the analyte based on the volume of reagent used and its known concentration. For example, 20 mL of 0.1 M NaOH neutralizes 25 mL of HCl, underscoring the importance of understanding molarity in calculation processes. This calculation is vital for achieving accurate results in pharmaceutical quality control and other analytical applications.
- Common Mistakes to Avoid: Be vigilant about frequent errors in the procedure, such as improper rinsing of the burette or overshooting the endpoint, as these can lead to inaccurate results. Conducting multiple trials and averaging the results can bolster reliability.
Titration, which utilizes a titrant and titrate, is a fundamental technique for various analytical procedures in research, industry, and environmental monitoring, making it a critical skill for pharmaceutical lab managers. With JM Science Inc.'s , including HPLC products and Karl Fischer reagents, you can significantly enhance your laboratory's efficiency and precision.
Troubleshoot Common Titration Issues
Common issues encountered during titration and their solutions include:
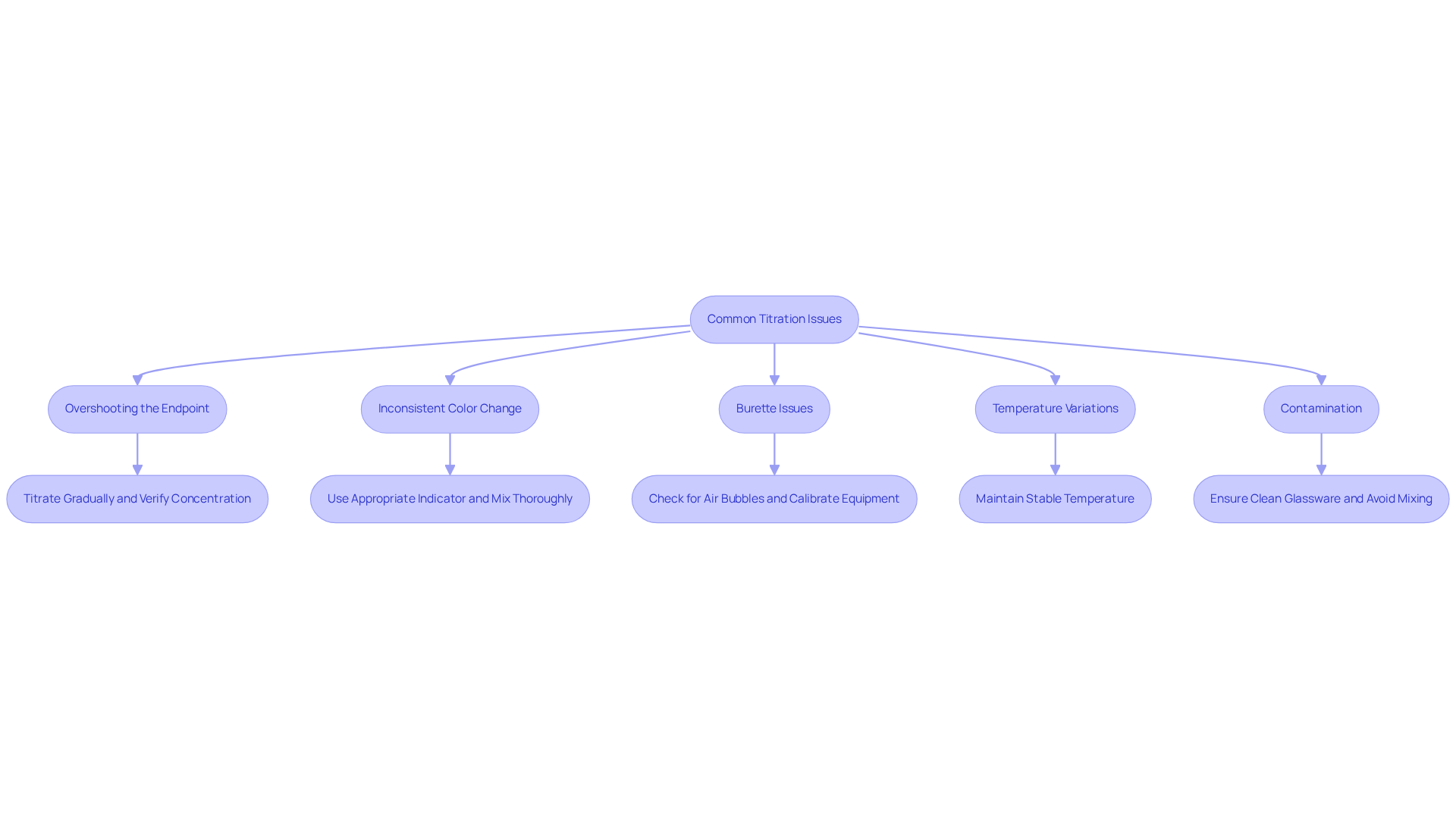
- Overshooting the Endpoint: Adding too much titrant can lead to inaccurate results. To avoid this, titrate by gradually adding the titrant as you near the conclusion. Performing a preliminary rough measurement can assist in estimating the conclusion more precisely. Research indicates that approximately 30% of laboratories report exceeding targets in analyses, underscoring the significance of precise methods. As noted by Kuldeep M., a Senior Technical Research Associate, "Verifying the concentration of the solutions utilized in the analysis is crucial to prevent exceeding the target point."
- Inconsistent Color Change: Inconsistent color change makes it difficult to accurately detect the endpoint when using a titrant and titrate. Ensure that the indicator used is appropriate for the specific titration type. Additionally, verify that the solutions are mixed thoroughly to facilitate a consistent color transition. Robert H. Grubbs, a Chemist, emphasizes that "accurate measurements are the foundation of good science," highlighting the importance of proper technique.
- Burette Issues: If the burette fails to deliver the titrant and titrate consistently, check for air bubbles in the tip or ensure that the stopcock is functioning correctly. Regular maintenance and calibration of equipment can prevent these issues. Performing regular calibration checks is vital to ensure accuracy in measurements.
- Temperature Variations: Temperature fluctuations can significantly impact reaction rates and solubility. Conduct the process at a stable temperature to minimize variability and enhance the reliability of results. This control is essential to avoid chemical errors that can arise from unstable conditions.
- Contamination: Contaminated glassware can severely affect measurement results. Ensure all glassware is clean and free from residues. Wash tools with the substances being utilized to , a frequent cause of errors in volumetric analysis. Meticulous record-keeping is also crucial for identifying potential sources of error and improving reproducibility.
By being aware of these common issues and implementing the suggested solutions, you can significantly enhance the accuracy and reliability of your results when using a titrant and titrate.
Conclusion
Mastering the art of titration is essential for anyone involved in analytical chemistry. This quantitative method not only allows for the precise determination of an unknown substance's concentration but also showcases the importance of meticulous preparation and execution in laboratory settings. Understanding the roles of key components—such as the titrant, analyte, and endpoint—enables chemists to achieve reliable results, reinforcing the technique's significance across various fields, including pharmaceuticals and environmental testing.
The article provides a comprehensive overview of the titration process, detailing the necessary materials and equipment, as well as a step-by-step procedure to ensure accuracy. Key insights such as the importance of stoichiometry, proper calibration, and common pitfalls to avoid serve as invaluable guidance for both novice and experienced practitioners. By addressing issues like overshooting the endpoint and ensuring consistent color changes, the article equips readers with the tools needed to troubleshoot and refine their techniques effectively.
In conclusion, embracing the principles of titration transcends mere protocol adherence; it fosters a mindset of precision and diligence in the laboratory. By applying the best practices outlined and remaining vigilant against common errors, chemists can enhance their analytical skills and contribute to the advancement of their respective fields. Whether in research or industry, mastering titration techniques empowers professionals to deliver accurate and meaningful results, ultimately reinforcing the foundational role of this method in scientific inquiry.




