Overview
This article delineates the essential steps and considerations for mastering the titration of hydrochloric acid and sodium hydroxide, a fundamental process in analytical chemistry crucial for determining the concentration of unknown solutions. It underscores the significance of precise methodology and equipment, highlighting the necessity of employing appropriate indicators and meticulous measurement techniques. These elements are vital to circumvent common errors that may compromise the accuracy of results. By adhering to these guidelines, practitioners can enhance their proficiency in this critical analytical technique.
Introduction
Acid-base titration is a cornerstone of quantitative analytical chemistry, serving as a reliable method for determining the concentration of unknown solutions. This technique, which involves the precise neutralization of an acid with a base, is fundamental not only in laboratories but also crucial in industries such as pharmaceuticals, where accuracy directly impacts product quality and safety. As titration techniques evolve, the integration of advanced tools and methodologies is reshaping how chemists approach this essential process.
Mastering acid-base titration is vital for professionals aiming to enhance their analytical skills and ensure the integrity of their results. Understanding the basic principles and troubleshooting common issues can significantly elevate one’s proficiency in this area. By embracing these advancements, chemists can ensure they remain at the forefront of analytical excellence.
Understand the Basics of Acid-Base Titration
Acid-base neutralization serves as a fundamental quantitative analytical method, crucial for determining the concentration of unknown acidic or basic mixtures by neutralizing them with a reagent of known concentration. This technique is particularly vital in analytical chemistry, where precision is paramount. Typically, the process involves a strong acid, specifically hydrochloric acid, and sodium hydroxide, which are both strong substances. The endpoint of the titration is marked by a distinct color change, commonly utilizing phenolphthalein, which transitions from colorless to pink as the solution shifts from acidic to slightly basic.
The stoichiometric relationship governing this reaction is encapsulated by the equation: hydrochloric acid and sodium hydroxide react to form NaCl and H2O. This signifies that one mole of hydrochloric acid and sodium hydroxide reacts with one another to produce one mole of sodium chloride and water. This straightforward yet powerful reaction underpins numerous applications across various fields, including pharmaceuticals, where it is essential for quality control and purity analysis. As Jessica Clifton, Director at ReAgent, emphasizes, "It may be used to analyse the purity or content of a substance, and for quality control purposes."
Recent statistics underscore the versatility of these methods, showcasing their application in diverse areas, such as assessing the acidity of waste vegetable oil and testing for diabetes through glucose detection in urine. These applications highlight the significance of measurement techniques in ensuring the quality and safety of pharmaceutical products. Moreover, advancements in measuring techniques, including the AQ-300 Coulometric Karl Fischer device and the AQV-300 Volumetric Karl Fischer apparatus from JM Science, are transforming laboratory practices. The AQ-300 provides enhanced sensitivity and precision for moisture determination, while the AQV-300 delivers , both ensuring compliance with the Japanese Pharmacopoeia.
Expert insights further emphasize the importance of meticulous methodology in measurement processes. Common errors, such as the rapid addition of titrant and inappropriate indicator selection, can lead to substantial inaccuracies in results. A case study on prevalent mistakes in acid-base analyses identified pitfalls such as these, underscoring the necessity for chemists to exercise caution and vigilance in their methodologies.
In summary, mastering the measurement of hydrochloric acid and sodium hydroxide solution is essential for laboratory managers in the pharmaceutical sector. By comprehending the principles, methodologies, and potential mistakes associated with acid-base analysis, professionals can ensure dependable and precise analytical outcomes, thereby enhancing the quality of their work and contributing to progress in the field.
Gather Required Materials and Equipment
To successfully conduct a titration involving hydrochloric acid and sodium hydroxide, several essential materials and equipment must be utilized. A 50 mL burette is required for accurately dispensing the titrant (NaOH), while a 25 mL pipette is essential for gauging the volume of the HCl liquid. The process also necessitates a 250 mL conical flask to hold the acid mixture during measurement. Indicators, such as phenolphthalein or methyl orange, play a crucial role in determining the endpoint through a visible color change. Additionally, distilled water is important for rinsing equipment and diluting solutions as needed. To enhance visibility of the color change during the process, placing a white tile under the flask is recommended. Safety equipment, including goggles and gloves, is crucial when handling corrosive substances. Furthermore, consider utilizing an E-burette, which features a battery indicator to display charging status, thereby improving accuracy and simplicity in .
Alongside these materials, it is vital to perform calculations for the molarity of the acid based on the amounts of HCl and sodium hydroxide used. As Joseph Louis Gay-Lussac noted, "This groundbreaking innovation marked a significant milestone in analytical chemistry, allowing scientists to measure and dispense liquids with exceptional accuracy." This quote underscores the importance of precision in measurement processes.
Moreover, organized data gathering is crucial for documenting the initial and final volumes of HCl and NaOH, as well as pH values before and after the titration. This practice not only aids in calculating hydrogen ion concentration but also exemplifies the application of theoretical concepts in real-world situations. By employing these materials and practices, one can ensure precise measurements while fostering a secure and effective laboratory environment, in accordance with best practices in acid-base analysis.
Execute the Titration Procedure
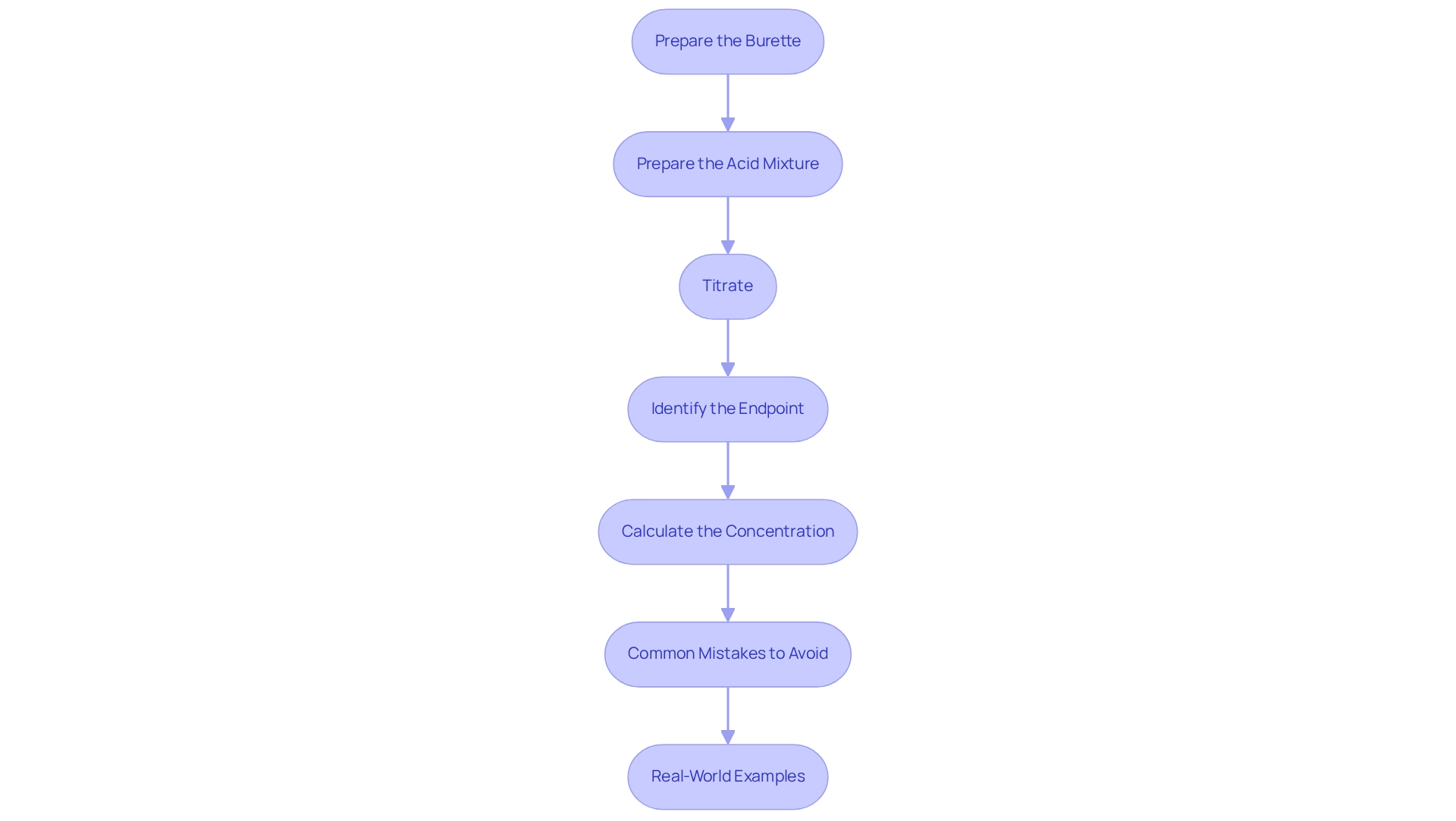
To carry out the titration of hydrochloric acid and sodium hydroxide, follow these detailed steps:
- Prepare the Burette: Start by rinsing the burette with distilled water, followed by a small quantity of the sodium hydroxide liquid to be used. Fill the burette with NaOH and record the initial volume accurately.
- Prepare : Measure 25 mL of HCl liquid using a pipette and transfer it to a clean conical flask. Add 2-3 drops of phenolphthalein indicator to the acid mixture, which will aid in visualizing the endpoint.
- Titrate: Position the conical flask on a white tile beneath the burette. Slowly introduce sodium hydroxide to the hydrochloric acid while continuously swirling the flask to guarantee complete mixing of hydrochloric acid and sodium hydroxide. As you near the endpoint, add the sodium hydroxide drop by drop to prevent overshooting.
- Identify the Endpoint: The endpoint is marked by a color change; the mixture will shift from colorless to a faint pink that lasts for about 30 seconds. At this moment, note the final volume of sodium hydroxide in the burette.
- Calculate the Concentration: To determine the concentration of the HCl solution, use the volume of NaOH dispensed along with its known concentration. Apply the formula: C1V1 = C2V2, where C represents concentration and V represents volume.
Common Mistakes to Avoid: Ensure that the burette is free of air bubbles, as this can lead to inaccurate measurements. Additionally, using high-quality consumables is crucial for maintaining sample integrity, which directly impacts the reliability of your results. Consistently swapping molecular sieves every six weeks is essential, as this method aids in preserving precision during measurement processes, in accordance with industry standards.
Real-World Examples: In laboratory environments, successful endpoint determination frequently depends on reliable practices and superior reagents. Case studies, including those from METTLER TOLEDO, have demonstrated that using high-quality consumables greatly enhances the precision of result measurements, underscoring the significance of quality in laboratory analyses. High-quality consumables not only improve the reliability of results but also guarantee that the endpoint is established precisely, which is essential for successful analytical outcomes.
Troubleshoot Common Titration Issues
Common problems during the process can significantly affect precision and dependability. Addressing these issues is crucial for achieving reliable measurement results. Here are some prevalent challenges and effective troubleshooting strategies to consider:
- Inconsistent Endpoint: Difficulty in determining the endpoint may arise from using an outdated indicator. Ensure the indicator is fresh and well-mixed, and consider switching to a more suitable indicator if necessary.
- Bubbles in the Burette: Air bubbles can skew measurements. To avoid this, fill the burette correctly and gently tap it to dislodge any trapped air.
- Over-titration: If surplus reagent is added, note the volume utilized and repeat the process for accuracy. Performing a preliminary measurement beforehand can assist in estimating the endpoint more precisely.
- Contamination: Residues from previous solutions can compromise results. Always clean glassware thoroughly and rinse with distilled water before use to ensure purity.
- Temperature Effects: Conduct experiments at room temperature, as fluctuations can alter reaction rates and solubility, leading to inconsistent results.
Participating in comprehensive training and remaining mindful of potential obstacles will equip chemists to navigate the challenges of acid-base experiments successfully. A thorough analysis of results from repeat trials is essential to address discrepancies and enhance accuracy. For instance, a case study on procedural mistakes in acid-base analyses emphasized that factors such as the speed of reagent addition and endpoint identification greatly influence precision. As noted by , "Science progresses by a series of lucky guesses or brilliant insights made while on the lookout for errors." By adhering to proper techniques and preparation practices, laboratories can enhance the integrity and accuracy of their titration results.
Conclusion
Acid-base titration stands as an indispensable technique in quantitative analytical chemistry, crucial for determining the concentration of unknown solutions. Mastery of the fundamentals—ranging from stoichiometric relationships to the pivotal role of precision in methodology—empowers professionals to significantly enhance their analytical capabilities. The significance of proper equipment and materials cannot be overstated; they form the backbone of successful titration practices.
Moreover, addressing common challenges encountered during titrations is essential for achieving reliable results. By employing effective troubleshooting strategies, chemists can minimize errors and ensure the accuracy of their findings. As titration techniques evolve with advancements in technology, embracing these innovations becomes crucial for maintaining high standards in laboratory practices.
Ultimately, a thorough understanding of acid-base titration not only aids in achieving precise analytical outcomes but also contributes to the broader goal of ensuring quality and safety across various industries, particularly pharmaceuticals. By committing to continuous learning and improvement in this essential skill, chemists can uphold the integrity of their work and advance the field of analytical chemistry.




