Overview
This article underscores the critical importance of mastering infrared (IR) spectroscopy within the realm of pharmaceutical applications, particularly highlighting its indispensable role in drug development and quality control. By elucidating the methodologies of IR spectroscopy—especially through techniques such as Fourier Transform Infrared (FTIR) and the distinctions between Near-Infrared (NIR) and Mid-Infrared (MIR)—the article reveals how these methods provide essential insights into molecular structures and functional groups. Such insights are vital in enhancing the accuracy and reliability of pharmaceutical analyses, ultimately driving advancements in the field.
Introduction
Infrared spectroscopy stands as a cornerstone analytical technique within the pharmaceutical industry, providing profound insights into molecular structures and compositions. By harnessing the unique 'fingerprint' of molecules, this method facilitates the precise identification of functional groups, playing a critical role in drug development and quality assurance.
However, as the complexity of pharmaceutical compounds escalates, the challenge of accurately interpreting infrared spectra intensifies.
How can researchers and industry professionals adeptly navigate these intricacies to fully leverage the potential of IR spectroscopy in their applications?
Explore the Fundamentals of Infrared Spectroscopy
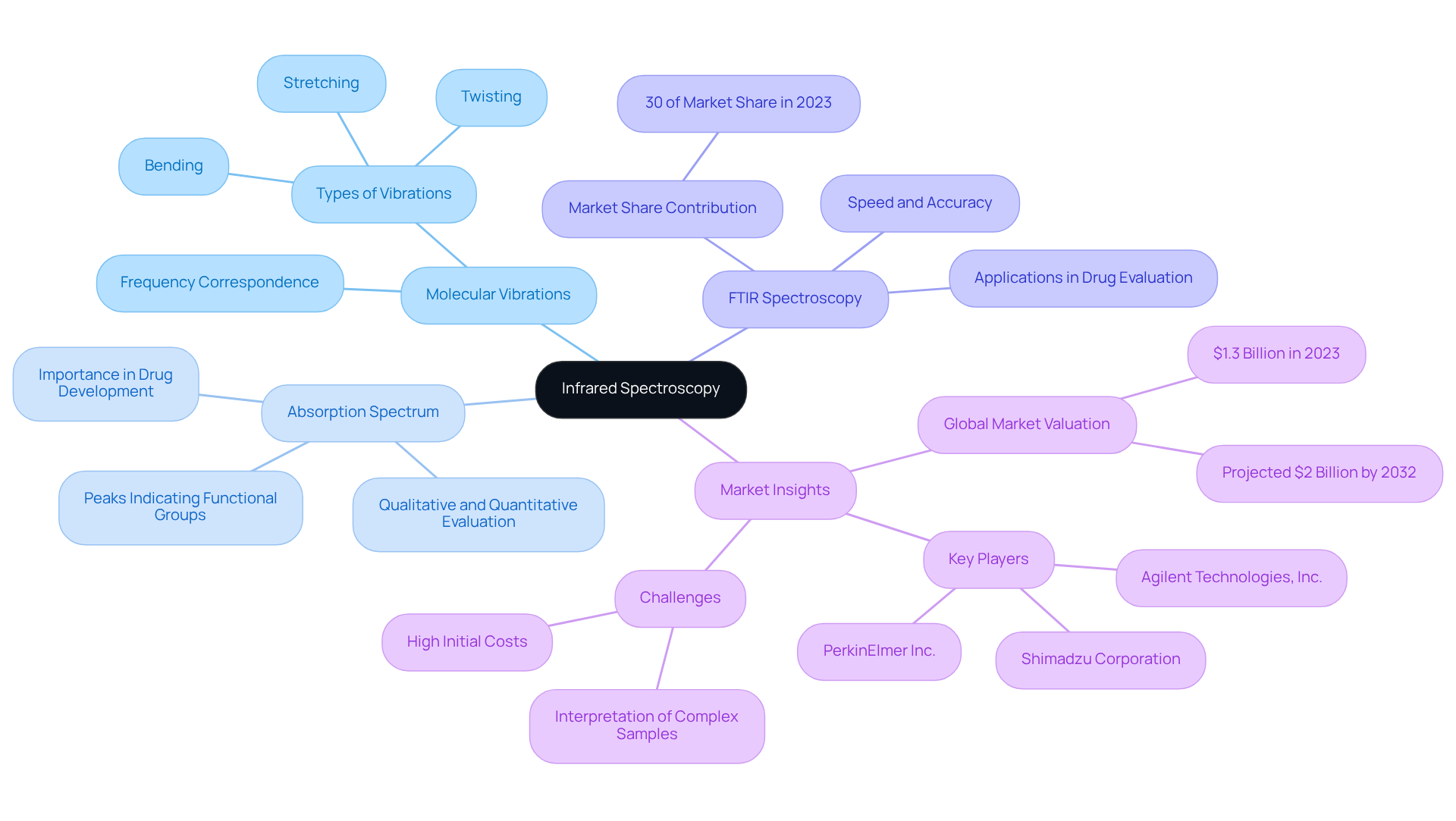
Infrared spectroscopy stands as a powerful analytical technique, measuring the absorption of infrared light by molecules and providing invaluable insights across various applications, particularly in pharmaceuticals. When infrared radiation interacts with a sample, it induces molecular vibrations that can be detected and analyzed. The resulting spectrum serves as a unique 'fingerprint' of the molecular structure, facilitating the identification of functional groups and molecular composition. Key concepts include:
- Molecular Vibrations: Molecules vibrate at specific frequencies that correspond to the energy of the infrared light. These vibrations manifest as stretching, bending, or twisting motions, each offering critical information about the molecular structure.
- Absorption Spectrum: The produced spectrum displays peaks at wavelengths where specific bonds absorb infrared light, indicating the presence of particular functional groups. This characteristic is essential for qualitative and quantitative evaluation in drug development and quality control.
- Fourier Transform Infrared (FTIR) Spectroscopy: This widely utilized method enhances the speed and accuracy of IR measurements by converting time-domain data into frequency-domain spectra. FTIR analysis has proven highly effective in drug evaluation, with research suggesting it can accurately recognize and measure active medicinal ingredients, contributing to approximately 30% of the market share in 2023. The global infrared analysis market is projected to reach around $2 billion by 2032, underscoring its increasing significance in the industry.
As Sarah Moore observes, "The drug industry makes great use of infrared analysis in its medication development processes." Understanding these fundamentals is vital for effectively employing in medical applications such as drug formulation and quality control. Nonetheless, challenges persist, particularly regarding the interpretation of infrared spectra for complex samples or mixtures, which can impede broader market penetration. Industry leaders emphasize that the integration of advanced techniques like FTIR is transforming analytical capabilities, rendering it indispensable for modern pharmaceutical research.
Differentiate Between NIR and MIR Spectroscopy
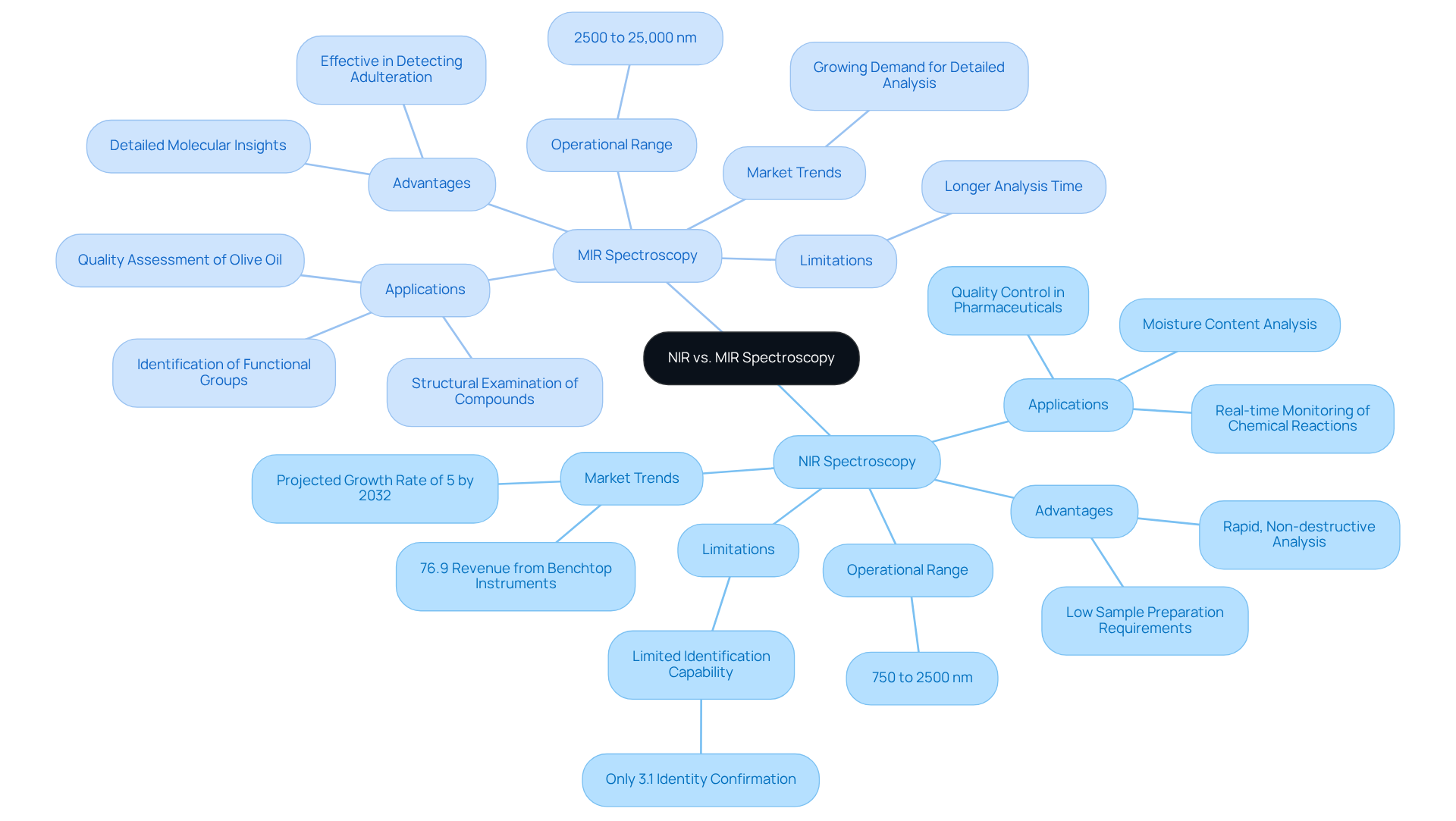
[Near-Infrared (NIR) and Mid-Infrared (MIR) spectroscopy](https://researchgate.net/publication/43533029_Comparison_between_NIR_MIR_concatenated_NIR_and_MIR_analysis_and_hierarchical_PLS_model_Application_to_virgin_olive_oil_analysis) are essential tools in analytical chemistry, each operating within the IR spectroscopy range to serve unique purposes tailored to specific analytical needs.
- NIR Spectroscopy operates within the wavelength range of approximately 750 to 2500 nm. Renowned for its rapid, non-destructive analysis capabilities, it allows for bulk material assessments without extensive sample preparation.
- NIR is particularly effective in analyzing moisture content in pharmaceuticals, with studies demonstrating its ability to achieve a relative standard error of prediction lower than 1.55% for active compound quantitation.
- Furthermore, it plays a crucial role in monitoring chemical reactions in real-time, facilitating immediate adjustments in production processes, and ensuring quality control in raw materials, thereby enhancing product reliability.
- However, it is important to note that NIRS confirmed the identity of only 3.1% of the tested medicinal products, highlighting some limitations in its application for identifying active ingredients during pre-marketing evaluation.
On the other hand, Mid-Infrared (MIR) Spectroscopy operates within the IR spectroscopy range of 2500 to 25,000 nm and provides detailed insights into molecular vibrations.
- This technique is more suited for identifying specific functional groups in complex mixtures, which is crucial for characterizing pharmaceutical compounds.
- It also enables thorough structural examination of solid and liquid specimens, allowing for a comprehensive understanding of chemical compositions, and evaluating authenticity while detecting adulteration in products, as evidenced by its application in assessing extra virgin olive oil quality.
The decision between depends on the particular needs of the evaluation, including sample type, desired detail level, and speed of examination.
- Recent trends suggest an increasing preference for NIR due to its effectiveness in moisture evaluation and quality control, whereas MIR continues to be essential for thorough structural characterization.
- Notably, the benchtop type segment accounted for more than 76.9% of the overall revenue in 2022, reflecting its dominance in the market.
As the market for infrared measurement continues to grow, with forecasts suggesting a growth rate of 5% to around $2 billion by 2032, both methods will be crucial in enhancing drug evaluation and quality control.
- Significantly, a case study on the control analysis of a drug formulation using NIR demonstrated its effectiveness in quality control, further emphasizing the relevance of these technologies in the medicine sector.
Utilize IR Spectroscopy in Laboratory Applications
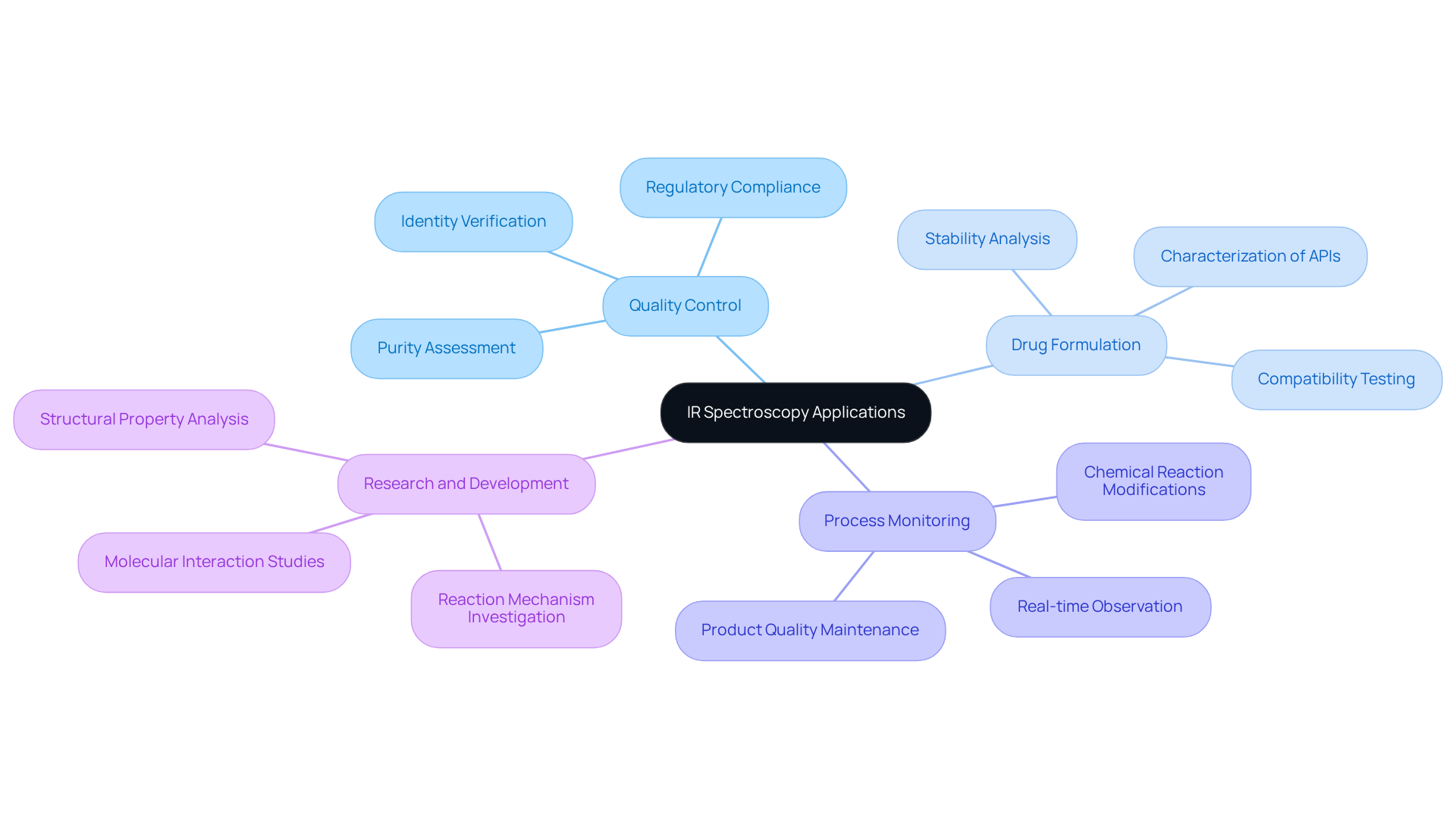
Infrared (IR) analysis is extensively utilized across various laboratory applications, particularly within the IR spectroscopy range in the medical field. Its significance is underscored by several key applications:
- Quality Control: IR spectroscopy is employed to verify the identity and purity of both raw materials and finished products. This technique is crucial for and ensuring compliance with regulatory standards.
- Drug Formulation: During the development of pharmaceutical formulations, the IR spectroscopy range plays a vital role in characterizing active pharmaceutical ingredients (APIs) and excipients. This ensures their compatibility and stability, which are essential for effective drug development.
- Process Monitoring: In manufacturing settings, IR analysis enables real-time observation of chemical reactions. This capability allows for timely modifications to be implemented, thereby upholding product quality.
- Research and Development: Researchers leverage IR analysis to investigate molecular interactions, reaction mechanisms, and the structural properties of new compounds. This exploration is fundamental to advancing pharmaceutical science.
These applications highlight the versatility of infrared analysis as a critical tool in ensuring the efficacy and safety of pharmaceutical products.
Implement Effective Techniques for IR Spectroscopy Analysis
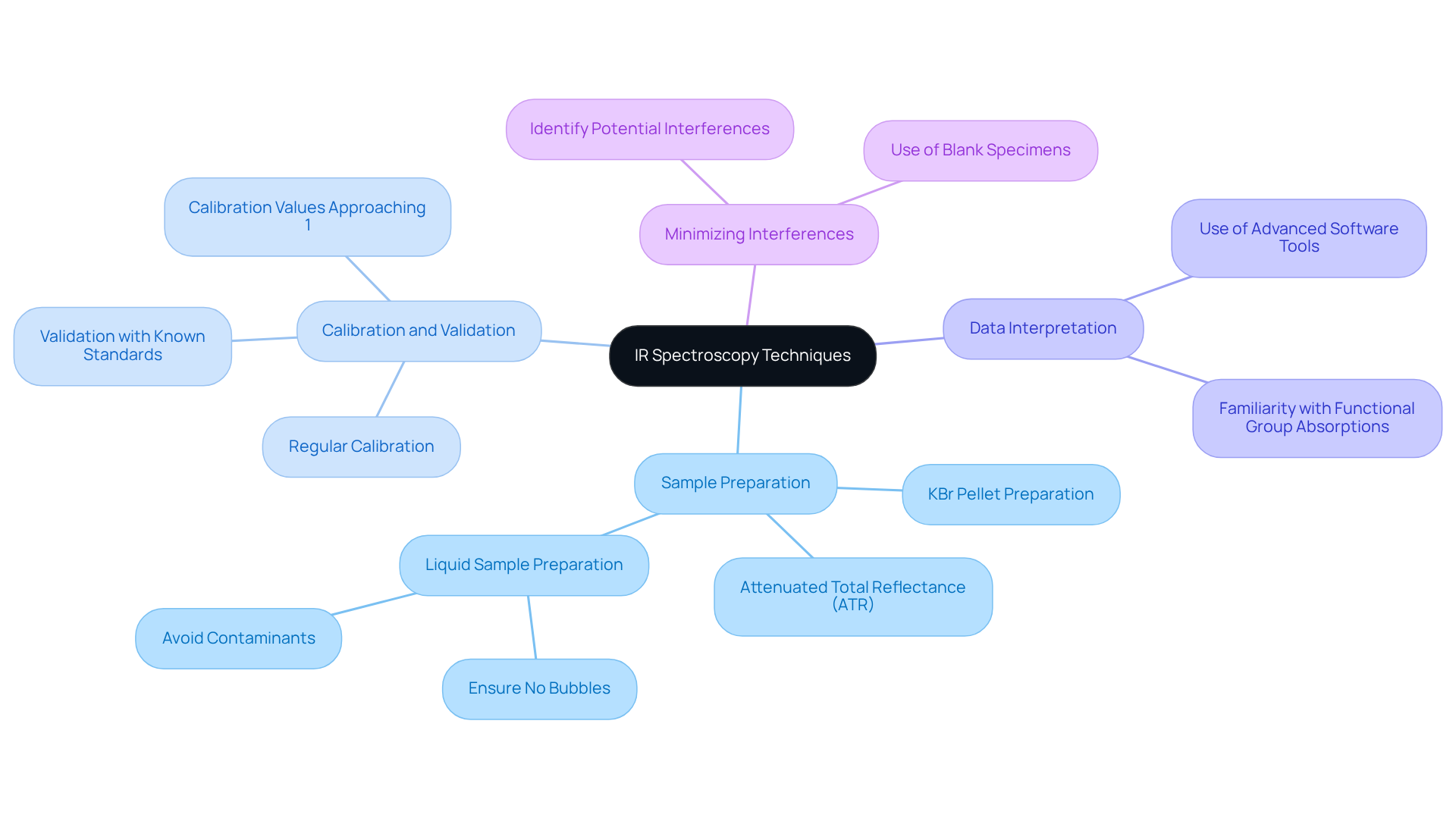
To achieve accurate and reliable results in the IR spectroscopy range analysis, it is imperative to consider effective techniques that enhance the quality of your evaluations.
Sample Preparation is crucial for optimal results. For solid materials, employing techniques such as KBr pellet preparation or utilizing attenuated total reflectance (ATR) can significantly improve spectral quality. When dealing with liquid samples, it is essential to ensure they are to avoid interference in the evaluation process.
Calibration and Validation of the IR spectrometer are vital for maintaining accuracy. Regular calibration is necessary, and validation of methods should involve comparing results with known standards to ensure reliability. Recent findings indicate that calibration values approaching 1 signify strong linearity, which is essential for effective evaluation.
Data Interpretation requires familiarity with common functional group absorptions and spectral patterns. Utilizing advanced software tools for spectral examination can assist in pinpointing peaks and interpreting intricate spectra, thereby enhancing the overall precision of the results.
Minimizing Interferences is another critical aspect. Be vigilant about potential interferences from solvents or other elements in the mixture. Employ suitable blank specimens to eliminate background noise, ensuring that the results accurately represent the genuine composition of the specimen.
Implementing these techniques will significantly enhance the quality of analyses in the IR spectroscopy range, leading to more accurate and reproducible results in pharmaceutical applications. Laboratory managers emphasize that meticulous attention to sample preparation and calibration practices is fundamental for achieving the best outcomes in the IR spectroscopy range.
Conclusion
Understanding the intricacies of infrared (IR) spectroscopy is essential for its effective application in the pharmaceutical industry. This analytical technique not only provides a unique molecular 'fingerprint' through the absorption of infrared light but also plays a pivotal role in drug development, quality control, and research. By mastering the IR spectroscopy range, professionals can enhance their ability to analyze and ensure the safety and efficacy of pharmaceutical products.
The article highlights several key points, including:
- The fundamental principles of IR spectroscopy
- The differences between Near-Infrared (NIR) and Mid-Infrared (MIR) spectroscopy
- The practical applications of IR analysis in laboratories
NIR is noted for its rapid, non-destructive capabilities, particularly in moisture analysis, while MIR excels in providing detailed insights into molecular structures. Furthermore, effective techniques such as proper sample preparation, calibration, and data interpretation are crucial for achieving reliable results.
As the demand for precise and efficient analytical methods grows within the pharmaceutical sector, the significance of IR spectroscopy cannot be overstated. Embracing these techniques not only enhances drug formulation and quality control processes but also fosters innovation in pharmaceutical research. By staying informed on current trends and advancements in IR spectroscopy, professionals in the field can ensure they are equipped to meet the evolving challenges and requirements of the industry.




