Overview
The equivalence point in titration represents a pivotal moment in analytical chemistry: it is defined as the instant when the quantity of titrant added precisely neutralizes the analyte solution. This critical juncture signifies that the moles of titrant are equal to the moles of the substance being titrated. Understanding this concept is essential in quantitative analysis, as it profoundly influences the accuracy of measurements and outcomes across various domains.
Fields such as pharmaceuticals and environmental science rely heavily on precise concentration determinations for quality control and regulatory compliance. Thus, mastering the concept of the equivalence point is not merely academic; it is a foundational element that underpins the integrity of scientific inquiry and practice.
Introduction
In the realm of analytical chemistry, the equivalence point in titration serves as a pivotal concept that effectively bridges theoretical knowledge with practical application. This critical juncture signifies the moment when titrant and analyte react in perfect stoichiometric balance, allowing chemists to determine concentrations with remarkable precision. The implications of accurately identifying this point resonate across various industries, from pharmaceuticals to environmental monitoring.
As titration techniques evolve, understanding the nuances of the equivalence point becomes essential for laboratory professionals striving for excellence in their analytical processes. This article delves into the significance of the equivalence point, exploring its:
- Definition
- Methodologies for determination
- Vital role in real-world applications
While also highlighting the innovative solutions offered by industry leaders like JM Science Inc.
Defining the Equivalence Point in Titration
The neutralization stage in the titration process represents a pivotal moment, defined as the instant when the volume of titrant added is precisely sufficient to fully neutralize the analyte solution. At this juncture, the moles of titrant are equivalent to the moles of the substance being titrated, ensuring a complete reaction. This concept is indispensable in quantitative analysis, enabling chemists to accurately ascertain the concentration of unknown solutions.
For instance, titrating 50.0 mL of 0.100 M hydrochloric acid with 0.200 M sodium hydroxide necessitates exactly 25 mL of NaOH to achieve the neutralization stage, exemplifying the precise calculations fundamental to titration processes.
Understanding this critical juncture is essential for professionals in chemistry and pharmaceuticals, as it directly impacts the precision of analytical outcomes. The weaker the acid or base, the greater the deviation of the pH from neutrality at the equivalence point, underscoring the necessity of selecting appropriate titrants. In scenarios involving mixtures with multiple acids, strong reagents are imperative to ensure complete reactions with all components, further emphasizing the need for meticulous planning.
This relevance is particularly pronounced for pharmaceutical lab managers tasked with ensuring the reliability of their analytical results.
JM Science Inc. offers a comprehensive array of premium titrators, Karl Fischer reagents, and various HPLC columns and accessories that are essential for achieving precise titration results. Their innovative solutions, including high-performance liquid chromatography (HPLC) columns and accessories, facilitate accurate analytical processes in pharmaceutical laboratories. Expert insights bolster this understanding; as noted by chemistry specialist Anne Marie Helmenstine, Ph.D., who specializes in analytical chemistry, "The neutralization stage occurs when the analyte solution is balanced."
Her expertise underscores the significance of this intersection in achieving dependable outcomes in quantitative analysis, particularly within the pharmaceutical domain.
Moreover, statistics indicate that proper sample handling is crucial in minimizing errors during analytical workflows. A case study titled "Sample Handling in Titration" revealed that inaccuracies often arise from sample size and homogeneity issues. Adhering to established protocols can significantly enhance the accuracy of measurement outcomes, ensuring that balance is achieved with precision.
This outcome is vital for pharmaceutical lab managers, as it directly correlates to the quality and reliability of their analytical processes.
In conclusion, the neutralization stage is not merely a theoretical concept; it is a cornerstone of quantitative analysis that informs practical applications in laboratories. By comprehending its definition and implications, professionals can refine their analytical techniques and contribute to advancements in research and healthcare, supported by the high-quality instruments provided by JM Science Inc.
The Role of the Equivalence Point in Acid-Base Reactions
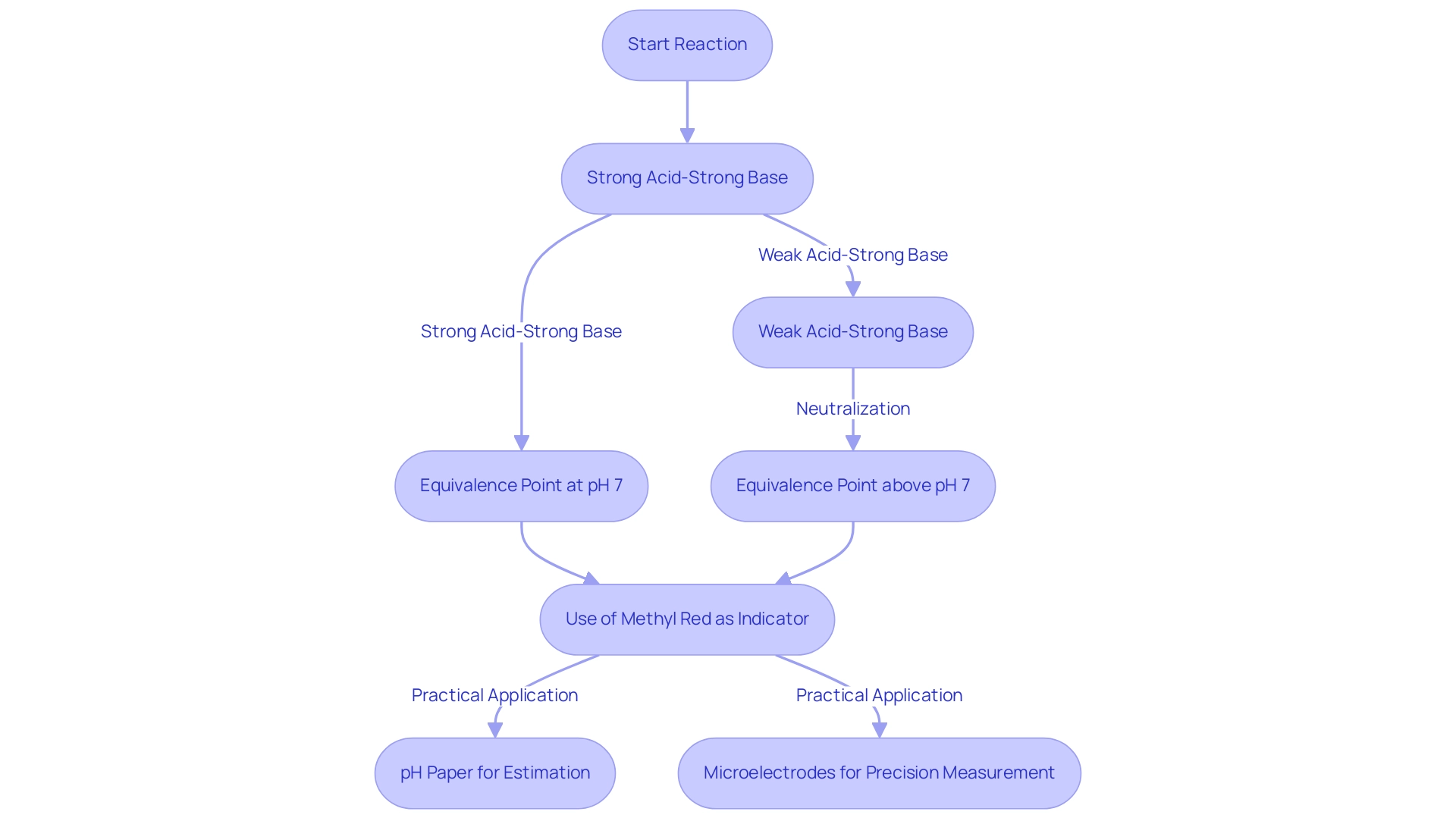
In acid-base reactions, the transition signifies the moment when the acid and base have reacted in stoichiometric proportions, marking a critical juncture in the process. For strong acid-strong base reactions, this stage typically occurs at a pH of 7, indicating a neutral solution. However, the scenario shifts when weak acids or bases are involved.
For instance, when neutralizing a weak acid with a strong base, the pH at the neutralization stage rises above 7 due to the formation of a weak conjugate base, which significantly impacts the curve of the process. Understanding the pH at this neutralization stage is vital for accurately analyzing the results of the reaction. Recent studies reveal that the pH at this stage can vary considerably depending on the specific acid-base pair utilized. The use of methyl red as an indicator for the analysis of NH with NaOH is particularly recommended, as its pKa aligns closely with the expected pH at the equivalence point, ensuring precise detection of this critical transition.
The practical implications of this knowledge are evident in various laboratory settings. A case study on the use of pH paper demonstrates its utility in estimating pH levels without the need for sophisticated equipment. This research establishes that pH paper, which changes color in response to the solution's acidity, offers a practical and economical method for pH estimation, making it accessible for both educational and professional laboratories.
Moreover, data indicate that microelectrodes can measure pH in extremely small liquid volumes, enhancing measurement precision in biochemical applications. Innovations in electrode design not only improve accuracy but also elevate the art of measurement to a new level of precision. Understanding these dynamics is crucial for laboratory managers and professionals aiming to refine their measurement procedures and ensure reliable outcomes.
Methods for Determining the Equivalence Point
Identifying the equivalence point in acid-base reactions is crucial for achieving precise analytical outcomes, and various techniques can be employed to accomplish this. The most prevalent method involves the use of pH indicators, which exhibit a distinct color change at specific pH levels. For instance, phenolphthalein is commonly utilized in strong acid-strong base analyses, transitioning from colorless to pink as the pH exceeds 8, signaling the endpoint.
In addition to pH indicators, pH meters offer a more accurate means of measuring pH variations throughout the process. By charting these measurements on a curve, analysts can visually identify the balancing stage with enhanced precision. Conductometric analysis provides another effective technique, tracking changes in electrical conductivity to facilitate precise identification of the balance point.
Moreover, spectroscopy and amperometry are relevant methods that can be utilized to determine the equivalence point, providing additional options for analysts.
Recent advancements in pH measurement technology have further improved the accuracy of results. For example, innovative pH indicators are being developed that deliver more distinct color changes, thereby enhancing the reliability of visual assessments. Additionally, a high-throughput method utilizing digital imaging from 96-well plates has been proposed, significantly decreasing analysis time while maintaining an average percent error of only 1.9%.
This method exemplifies the ongoing evolution in measurement techniques, illustrating how modern approaches can streamline laboratory processes.
Real-world applications of these methods are evident across various scientific fields, particularly in pharmaceutical laboratories where precise measurements are essential. By leveraging these advanced techniques, laboratories can ensure accurate and reliable results in their measurements, ultimately enhancing the quality of their analytical outcomes. JM Science Inc. distinguishes itself through its commitment to quality and customer support, offering exceptional pricing on products such as Karl Fischer reagents and Shodex HPLC columns, which cater to the evolving needs of the scientific community.
Equivalence Point vs. Endpoint: Key Differences
The concepts of balance and endpoint in titration are often conflated, yet they signify distinct phenomena. The theoretical stage represents the moment when reactants are present in stoichiometrically equivalent quantities, illustrating the equivalence point for the complete neutralization of the involved acid and base. In contrast, the endpoint is characterized by a noticeable change, typically a color shift in the solution due to the action of a pH indicator.
Ideally, these two points should coincide; however, discrepancies frequently occur, particularly when the selected measure does not align with the pH at the equivalence point.
Understanding this distinction is crucial for achieving accurate measurement results. For example, in scenarios involving weak acids, such as boric acid, traditional pH tests may fail to yield accurate readings due to low equilibrium constants. A case study on thermometric titration exemplifies this issue, demonstrating how monitoring temperature changes can effectively identify the balance stage, even when pH indicators are ineffective.
This method not only enhances accuracy but also provides a reliable alternative for determining endpoints in challenging situations, particularly in pharmaceutical applications where precision is paramount.
Recent findings underscore the necessity of aligning indicators with the neutralization pH to mitigate discrepancies. The logarithmic nature of the pH scale implies that each whole number change corresponds to a tenfold variation in hydrogen ion concentration, complicating the understanding of the equivalence point in relation to the endpoint. As this analytical technique finds application across diverse sectors—including pharmaceuticals, food and beverage industries, and environmental testing—ensuring accuracy in these measurements is essential.
Expert insights assert that "this process is more than a laboratory technique; it is a bridge connecting theoretical chemistry with practical applications in our world." Therefore, a comprehensive understanding of these concepts is vital for laboratory managers seeking to enhance the reliability of their analytical results. Furthermore, manual analyses, which require minimal equipment, are generally quicker than gravimetric methods, making them a practical choice for many laboratory environments.
The Role of Indicators in Identifying the Equivalence Point
Indicators are vital substances that exhibit color changes at specific pH ranges, serving as essential tools in acid-base reactions. They signal the endpoint of a reaction, ideally aligning with the equivalence point—the moment when the amount of titrant is stoichiometrically equivalent to the analyte. The selection of an appropriate indicator is crucial, as it directly influences the precision of the results.
For example, phenolphthalein is commonly employed in strong acid-strong base reactions due to its distinct transition at a pH of approximately 8.2 to 10.0. In contrast, methyl orange is preferred for strong acid-weak base reactions, changing color within a pH range of 3.1 to 4.4.
Nevertheless, challenges such as the leveling effect can obscure equivalence points when analyzing strong acids like sulfuric and phosphoric acids, thereby complicating the accuracy of results. Recent studies have highlighted the effectiveness of various measures in chemical analysis. For instance, research into natural indicators, such as those derived from guinea corn leaves, has yielded promising outcomes.
This research demonstrated that these natural markers can effectively indicate transition stages across diverse acid-base titrations, including strong acid versus strong base and weak acid versus weak base. The findings revealed that the natural substance not only provided accurate color changes but also proved to be cost-effective and environmentally friendly, establishing it as a viable alternative to traditional synthetic indicators.
Current trends in the selection of indicators underscore the importance of aligning the indicator with the expected pH at the equivalence point. The plumbagin solution, for example, has been recognized for its effectiveness in varying pH environments, exhibiting color changes that correspond with its functional pH range of 8.02 to 10.07. This adaptability makes it an invaluable tool for obtaining precise measurement results.
Professional insights emphasize the necessity of employing the correct indicator to minimize errors in the measurement process. As noted by Kapilraj Natkunarajah, "commonly utilized metrics for acid-base experiments are synthetic, and this work was aimed at identifying the eco-friendly natural metrics and determining their pKa values." This highlights a growing interest in enhancing measurement accuracy while considering environmental impacts.
Moreover, the linearity for the quantification of aprepitant was found to be 0.1 - 10 µg/mL, adding a quantitative dimension to the discussion on effectiveness.
In summary, the role of indicators in titration is paramount for accurately determining equivalence points. By staying informed about the latest advancements and trends in indicator selection, laboratories can enhance their analytical precision and contribute to more reliable scientific outcomes.
Understanding Titration Curves and the Equivalence Point
Titration curves are essential for visualizing the relationship between a solution's pH and the volume of titrant added. As titrant is introduced, the pH undergoes notable changes, often demonstrating a significant increase near the neutralization stage. This inflection point on the curve indicates that the reactants are reacting in stoichiometric proportions, a critical factor for precise analysis.
For instance, in strong acid-strong base reactions, the curve typically exhibits a sharp increase in pH near the transition, indicating a rapid change in acidity. Conversely, weak acid-strong base reactions display a more gradual slope, illustrating the subtler dynamics of the process.
Understanding the shape of the curve is vital for chemists aiming to determine the equivalence point accurately. Recent research underscores the importance of halting data collection after adding approximately 5 mL of titrant beyond the equivalence point, ensuring that the analysis remains focused and relevant. Furthermore, rinsing all glassware after use is crucial to prevent contamination and maintain accurate results.
In practical applications, non-aqueous analyses broaden the range of techniques available, enabling the examination of substances that are insoluble in water or involve very weak acids and bases. This versatility enhances the capacity to analyze complex mixtures, establishing this technique as a valuable method in various laboratory environments. A case study on non-aqueous analyses exemplifies how these techniques facilitate the investigation of intricate mixtures.
Moreover, the use of pH probes calibrated against standard solutions is essential for ensuring accuracy during experimental procedures. This calibration process guarantees that pH measurements accurately reflect true changes in the solution, thereby enhancing the reliability of results. Accurate measurement is particularly crucial in pharmaceutical applications, where precise calculations can significantly influence treatment outcomes.
At JM Science Inc., we offer a comprehensive array of premium analyzers, Karl Fischer reagents, HPLC solutions, and various HPLC columns and accessories that enhance the accuracy and efficiency of measurement processes. By analyzing curve data with our advanced instruments, laboratory experts can gain insights into the equivalence point during the neutralization stage, thereby improving decision-making in chemical evaluations. This understanding not only aids in achieving precise measurements but also contributes to advancements in research and healthcare applications.
Applications of the Equivalence Point in Real-World Scenarios
The equivalence point represents a fundamental concept in various scientific processes, with significant applications across pharmaceuticals, environmental science, and food chemistry. In the pharmaceutical sector, precise measurements are critical for determining the concentration of active ingredients in drug formulations. This process ensures quality control and guarantees compliance with stringent regulatory standards, which is vital for patient safety and the efficacy of medications.
For instance, the combination of tramadol and acetaminophen has demonstrated a synergistic analgesic effect in clinical evaluations, underscoring the importance of precise measurements in drug development.
JM Science Inc. offers a broad selection of high-quality titrators and Karl Fischer reagents essential for these analytical procedures. Their innovative solutions, including high-performance liquid chromatography (HPLC) columns and accessories, support pharmaceutical laboratories in achieving accurate and reliable results. The HPLC columns provided by JM Science, such as Shodex and CapcellPak, are designed for optimal performance, ensuring that laboratories can conduct precise analyses efficiently.
Environmental scientists utilize measurement techniques to evaluate water quality by quantifying the concentration of pollutants. This application is crucial for monitoring environmental health and ensuring compliance with environmental regulations. JM Science's HPLC solutions, including Shodex Refractive Index and Conductivity detectors, enhance the capabilities of environmental testing laboratories, providing reliable data for regulatory compliance.
In the food industry, tests are employed to assess the acidity of products, which plays a key role in flavor development and preservation. Professionals in these fields can achieve accurate measurements and improve product quality by understanding the equivalence point, leading to effective analysis.
Recent advancements in measurement techniques have further broadened their applicability. The establishment of Collaborative Industry Groups (CIGs), focused on themes such as Quality by Design and Continued Process Verification, is essential for refining titration processes in environmental science and food chemistry. These developments underscore the importance of staying informed about innovative techniques that can enhance analytical capabilities.
As Jason Walker, a consultant anaesthetist, observes, "It is uncommon to use comparison trials in medicine—normally a treatment which has more than its required effect is considered a useful property." This perspective highlights the practical uses of comparison trials in ensuring effective treatment outcomes.
In summary, the equivalence point is not merely a theoretical concept; it serves as a practical tool that underpins quality assurance and regulatory compliance across various scientific fields. JM Science Inc.'s commitment to quality and customer support, alongside its focus on innovation in scientific instrumentation, aligns with the critical importance of accurate measurements in these areas. Mastery of this concept is essential for professionals striving to ensure the integrity and safety of their products.
Conclusion
The equivalence point in titration stands as a cornerstone in analytical chemistry, effectively bridging the gap between theoretical knowledge and practical application. By grasping its definition, methodologies for determination, and real-world applications, professionals can significantly enhance their analytical processes, leading to more reliable results. The precise identification of the equivalence point is crucial for accurate concentration measurements, which hold immense importance across diverse industries such as pharmaceuticals, environmental monitoring, and food quality control.
Innovative techniques, including advanced pH indicators and modern titration technologies, have markedly improved the accuracy and efficiency of titration processes. The distinction between the equivalence point and the endpoint further underscores the necessity for meticulous planning and execution in titrations. By selecting suitable indicators and embracing advanced methodologies, laboratory professionals can effectively minimize errors and optimize their results.
Ultimately, mastering the equivalence point is essential for ensuring quality assurance and regulatory compliance in scientific fields. As titration techniques evolve, the unwavering commitment of industry leaders like JM Science Inc. to providing high-quality instruments and support empowers laboratories to achieve excellence in their analytical endeavors. Embracing these advancements not only bolsters the reliability of results but also contributes to ongoing progress in research and healthcare, making the understanding of the equivalence point an invaluable asset for professionals in the field.




