Overview
This article provides a comprehensive comparison of the roles of titrant and analyte in the titration process. The titrant is identified as a standard solution with a known concentration, while the concentration of the analyte remains unknown until determined through titration. This distinction is crucial for accurate quantitative analysis. The effectiveness of the titration hinges on the careful selection of titrants that react appropriately with the analyte. Such precision is essential for ensuring reliable results across various applications, particularly within the realms of pharmaceuticals and environmental testing.
Introduction
In the realm of analytical chemistry, titration emerges as a pivotal technique, indispensable for precisely determining the concentration of unknown solutions. This article explores the intricate world of titration, delving into its fundamental concepts, the critical roles of titrants and analytes, and the various methodologies that underpin this analytical process.
By understanding the nuances of equivalence points and examining the latest advancements in titration technology, readers will gain valuable insights into the application of this technique across industries, particularly in pharmaceuticals and environmental testing.
As the demand for accuracy and reliability in laboratory results continues to escalate, mastering the art of titration becomes increasingly vital for professionals committed to upholding the highest standards in their analytical practices.
Understanding Titration: Definitions and Key Concepts
Titration stands as a fundamental quantitative analytical technique employed to ascertain the concentration of an unknown solution, known as the analyte, by systematically adding a titrant—illustrating the essential distinction between titrant and analyte—until the reaction reaches completion. Central to this process are two critical concepts: the equivalence point and the endpoint. The equivalence point is defined as the moment when the quantity of titrant added is stoichiometrically equivalent to the amount of analyte present, reinforcing the distinction between titrant and analyte, while the endpoint is marked by a discernible change, typically indicated by a color shift due to an indicator.
Understanding these concepts is crucial for obtaining accurate measurement results and ensuring effective laboratory practices. For instance, volumetric analysis, particularly using the Hiranuma Aquacounter AQV-300 from JM Science, is widely utilized to determine water content in pharmaceutical samples, ranging from 100 ppm to 100%. This method underscores the importance of precise measurements in various applications, including pharmaceuticals and environmental testing, and complies with the Japanese Pharmacopoeia standards.
The AQV-300 also incorporates suitability tests that ensure its effectiveness in diverse pharmaceutical applications.
Similarly, the AQ-300 Coulometric Karl Fischer Titrator from JM Science plays a significant role in drug and medicine testing, providing precise water content analysis essential for quality control in pharmaceutical manufacturing. Recent studies have emphasized the importance of understanding volumetric analysis techniques in analytical chemistry. An experimental study titled 'Teaching Precipitation Titration Methods: a Statistical Comparison of Mohr, Fajans, and Volhard Techniques' introduced students to three prevalent titration methods.
The experiment involved quantifying potassium iodide (KI) and sodium chloride (NaCl) in different solutions, employing statistical analysis to compare the efficacy of each method. Results revealed that the Mohr method consistently yielded higher concentration values compared to the Volhard and Fajans methods, thereby enhancing students' practical experience in both analytical chemistry and statistical analysis. This case study illustrates the real-world applications of these techniques and the significance of statistical rigor in research environments.
Moreover, experts emphasize the critical nature of the equivalence point and endpoint in the analysis of titrant versus analyte. Siegbert Pantel notes that methods based on electrochemical techniques (pH-stat, potentiostat, amperostat, and biamperostat) and on spectrophotometric and luminescence techniques (absorptiostat, fluorostat, and luminostat) are described, with their applications summarized. Understanding these concepts not only aids in achieving accurate results but also enhances the reliability of laboratory practices.
As these techniques continue to evolve, recent advancements have introduced innovative methods that enhance precision and efficiency, further solidifying the role of this process in modern analytical chemistry. Additionally, examining the effect of increasing the number of measurements on statistical values and the concept of normal distribution can offer further insights into the reliability of results.
The Roles of Titrant and Analyte in Titration
In titration, the relationship between titrant and analyte is established, where the titrant serves as the reagent with an accurately determined strength, added to the analyte, which possesses an unknown strength. This interaction is crucial, as the titrant reacts with the analyte in a controlled manner, enabling chemists to accurately determine the analyte's amount based on the volume of titrant utilized. The effectiveness of this process hinges on the careful selection of titrants, particularly concerning their concentration, which directly influences the accuracy of analyte determination.
Recent studies have highlighted significant variations in NaCl percentages across different testing methods, with the Fajans method yielding the highest results. This underscores the importance of method selection in achieving reliable outcomes. For instance, in chloride quantification, Park reported that the Mohr method demonstrates greater precision compared to the Fajans method, emphasizing the necessity for analytical chemists to choose their measurement techniques wisely.
A practical illustration of titrant and analyte interactions can be observed in the experiment titled 'Studying Equilibrium in the Chemical Reaction between Ferric and Iodide Ions.' This experiment employs argentometric analysis to ascertain the concentrations of ferric and iodide ions, making it accessible for first-year students while also allowing for advanced extensions. Such studies not only enhance a deeper understanding of chemical equilibrium but also illustrate the critical roles that titrant and analyte play in analyses.
Moreover, recent findings indicate that the addition of 0.1 μM sulfide ions to AgNPs-CDs resulted in a decrease in absorbance intensity at 417 nm, highlighting the quantitative impact of titrants in analytical processes.
Expert insights further illuminate the dynamics of titrant and analyte interactions. Analytical chemists assert that the selection of reagent can greatly influence the results of volumetric analysis, especially in pharmaceutical laboratories where accuracy is paramount. Current trends indicate a growing focus on innovative measurement techniques that enhance the reliability and efficiency of these interactions, reflecting JM Science's commitment to meeting the evolving needs of the scientific community.
In summary, the functions of the titrant and analyte in titration processes are foundational to accurate quantitative analysis. Understanding their interactions not only aids in achieving precise measurements but also fosters advancements in laboratory practices, particularly within the pharmaceutical sector.
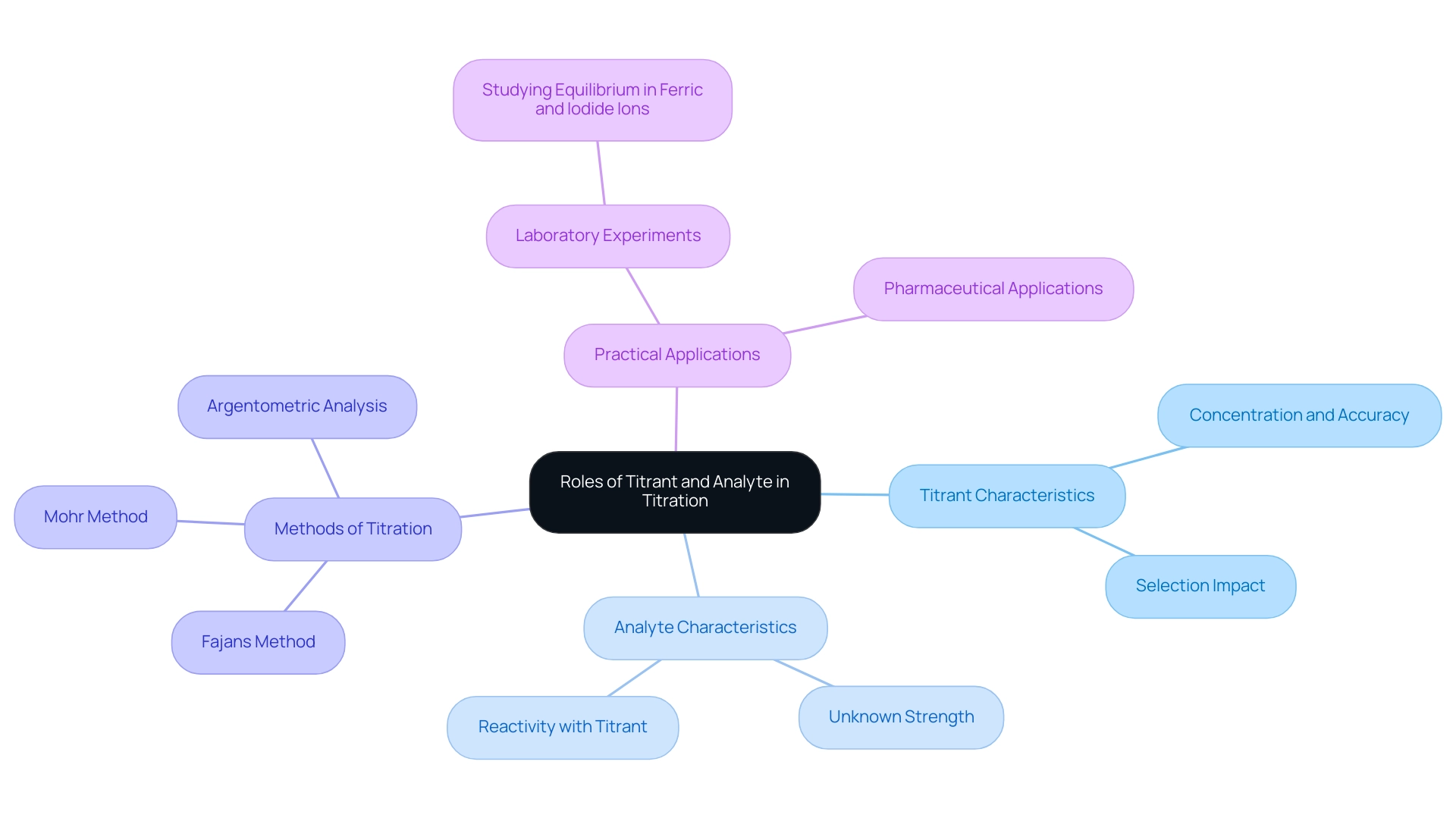
Key Differences Between Titrants and Analytes
In analytical chemistry, the distinction between a reagent and an analyte primarily hinges on their respective concentrations. The differentiation between titrant and analyte is pivotal; the titrant is a standard solution with a precisely known concentration, whereas the analyte's concentration is generally unknown and serves as the focal point of the analysis. This fundamental difference is crucial, as it directly influences both the analytical process and the accuracy of the results obtained.
The selection of titrants is contingent upon their reactivity with the analyte, underscoring the significance of the titrant-analyte interaction to ensure that reactions proceed to completion with specificity. For example, when titrating phosphoric acid, the apparent pKa values suggest that it is so close to 0.01 that only the second equivalence point becomes detectable. This scenario highlights the necessity for meticulous selection of titrants capable of effectively reacting with the analyte, emphasizing the importance of comprehending titrant-analyte interactions at various stages of the analytical process.
Choosing the appropriate reagent transcends mere procedural compliance; it is a strategic decision that can significantly impact the titration's outcome. Selecting a reagent that aligns with the reactivity profile of the titrant and analyte is essential for achieving accurate and reliable results. For instance, when the analyte is a weak acid, employing a strong base reagent may be preferred to ensure a definitive endpoint.
Furthermore, the quantity of reagent required to reach the equivalence point can be calculated using the moles of the analyte and the concentration of the reagent, providing a quantitative basis for the process.
Data regarding the selection criteria for reagents in analytical chemistry accentuate the necessity of understanding the chemical properties of both titrant and analyte. Expert opinions consistently emphasize that the right titrant can enhance the precision of the measurement process, thereby improving the overall quality of analytical results. As noted by David Pierre, "The concentration of the unknown acid is found by using this mathematical model," which underscores the importance of accurate calculations in determining analyte concentrations.
This relevance is particularly pronounced in pharmaceutical applications, where precise measurements are critical for product development and quality control.
In summary, the differences in the titrant-analyte relationship extend beyond mere definitions; they embody a strategic approach to analytical chemistry that prioritizes accuracy and specificity in measurement processes. JM Science's commitment to quality and customer support further illustrates how these factors influence the selection of titrants and the overall analytical process, ensuring that laboratories can achieve reliable results in their analyses.
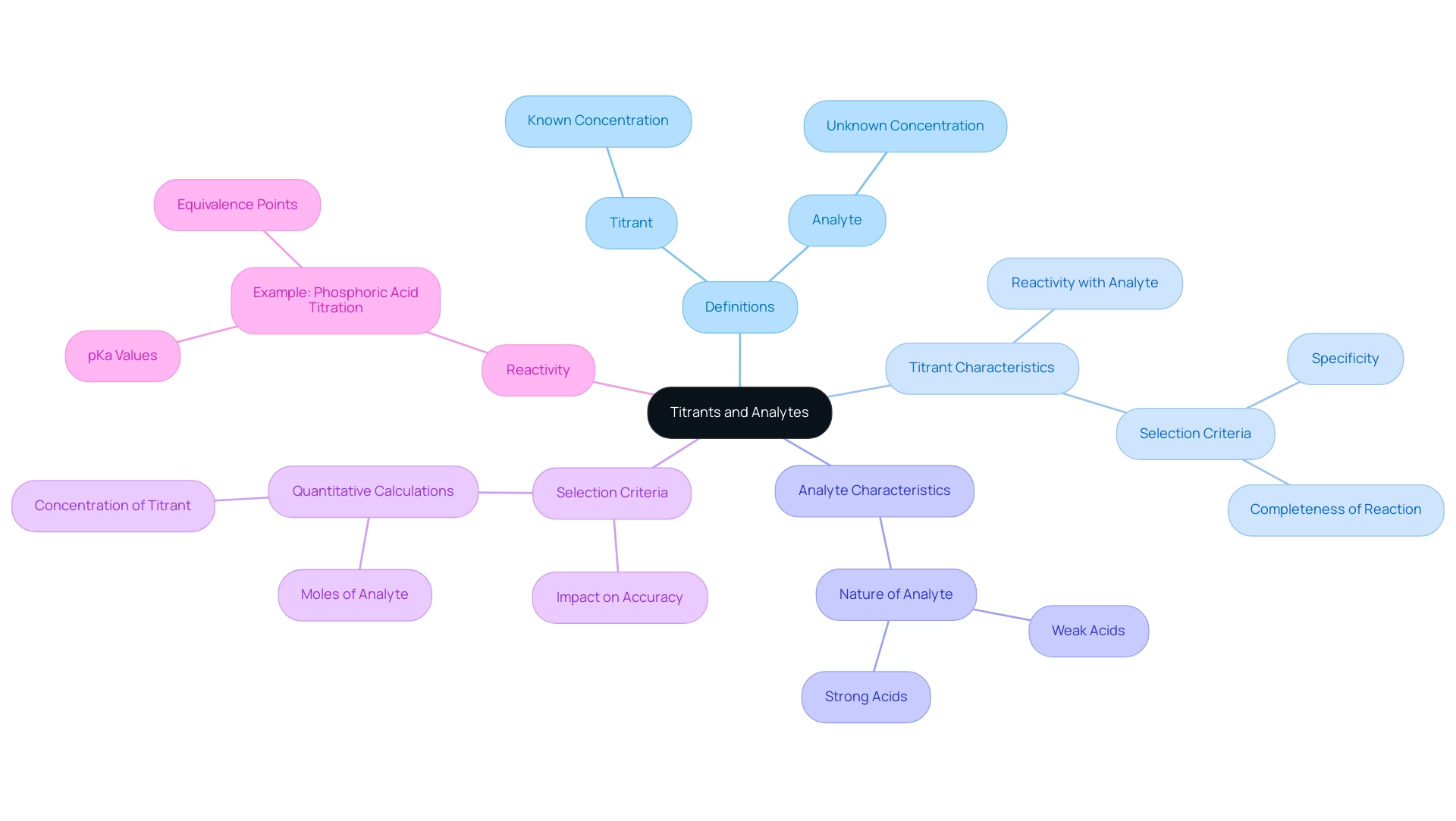
Types of Titration: Contextualizing Titrants and Analytes
Titration processes are fundamental analytical techniques, categorized into several distinct types:
- Acid-base methods
- Redox methods
- Complexometric methods
- Precipitation methods
Each category is characterized by specific titrant vs analyte pairs, chosen based on the underlying chemical reactions involved. For instance, in acid-base reactions, a strong acid typically reacts with a strong base, enabling an accurate assessment of pH variations.
Conversely, redox procedures involve titrants that act as oxidizing agents, reacting with reducing agents to facilitate analysis. Choosing the right titrant vs analyte pair is essential for obtaining accurate results. Expert insights emphasize that the compatibility of these pairs can significantly influence the measurement process's outcome. For example, using a titrant that does not react effectively with the analyte can lead to erroneous conclusions, underscoring the importance of understanding titrant vs analyte dynamics in laboratory settings.
As Laina L. Maines noted, "We have developed a problem-based experiment to improve student learning of these concepts, and student surveys indicate this goal was achieved," highlighting the educational importance of these relationships.
Statistics indicate that measurement techniques used for analyzing bismuth subsalicylate in over-the-counter stomach relief products consistently comply with United States Pharmacopeia (USP) standards, where the permissible range of active ingredient amount is upheld within 90-110% of the label value. This emphasizes the reliability of quantitative analysis methods in pharmaceutical applications.
In practical applications, acid-base analyses are frequently utilized in pharmaceutical examination to determine the concentration of active ingredients, while redox methods are essential for assessing the oxidation states of compounds. Recent advancements in these methods have introduced more sophisticated techniques, enhancing accuracy and efficiency in experimental settings.
Current trends indicate a growing preference for automated titration systems, which streamline the process and reduce human error. These advancements are particularly beneficial in high-throughput settings where precision and speed are paramount. JM Science Inc. supports these advancements by offering a wide range of premium titrators, including Karl Fischer titrators and reagents, as well as HPLC solutions featuring Shodex and capcell Pak columns.
Their dedication to quality is further demonstrated by their extensive technical support with every inquiry, enhancing the customer service experience for professionals in the field.
Overall, comprehending the different kinds of volumetric analysis and their specific reagents and substances is crucial for professionals in the field. By leveraging this knowledge, they can ensure the integrity of their analytical results and contribute to the advancement of pharmaceutical research and development. Furthermore, JM Science Inc. differentiates itself in the market through its commitment to quality and customer support, as illustrated in the case study on customer support and value proposition, which emphasizes exceptional pricing on HPLC components and extensive support resources.
Customer testimonials highlight the effectiveness of JM Science's products, reinforcing their reputation as a trusted provider in the scientific community.
Practical Applications of Titration in Laboratories
Titration stands as an essential analytical technique employed across various laboratory settings, particularly in pharmaceutical labs, where the interplay between titrant and analyte is crucial for determining the concentration of active ingredients in drug formulations. This method not only ensures that products meet stringent regulatory standards for potency and purity but also bolsters quality control processes vital for maintaining product consistency and adherence to industry standards. For instance, it is instrumental in achieving the desired AUC/MIC ratio of 400–600 for vancomycin dosing in treating methicillin-resistant Staphylococcus aureus infections, underscoring its significance in pharmaceutical applications.
JM Science Inc. offers a comprehensive selection of premium titrators, including Karl Fischer models and reagents, enhancing the measurement process to ensure accuracy and reliability in pharmaceutical applications. Their innovative solutions, such as Shodex Refractive Index and Conductivity detectors, high-performance liquid chromatography (HPLC) columns, and accessories, further empower laboratories in upholding high standards of quality control.
Beyond pharmaceutical applications, these techniques are widely utilized in environmental testing to monitor pollutants, ensuring compliance with environmental regulations. For example, specific analytical methods can evaluate the concentration of contaminants in water samples, providing essential information for environmental safety. Similarly, in the food sector, methods are employed to examine food items, guaranteeing conformity to safety standards and regulatory requirements.
Real-world case studies illuminate the practical applications of titration in pharmaceutical laboratories. A notable example involves determining solution strength, where a known volume of a solution with a known potency is added until the reaction reaches completion. This process enables scientists to accurately calculate unknown concentrations, which is crucial for analyzing the titrant vs analyte relationship in both experimental chemistry and quality control in industrial settings.
This case study underscores the importance of precise measurement in ensuring that pharmaceutical products are formulated correctly and meet quality standards. Industry professionals recognize the significance of this process in ensuring drug potency and purity. As Eric P. Borrelli from the University of Rhode Island College of Pharmacy states, "The views expressed are those of the authors and do not necessarily reflect the position or policy of the United States Department of Veterans Affairs." Expert opinions highlight that this technique not only aids in drug formulation compliance but also enhances overall quality assurance processes within pharmaceutical laboratories.
By employing analytical methods and utilizing JM Science's advanced instruments, including HPLC fittings and manual injection valves, labs can effectively monitor and control the quality of their products, ultimately contributing to safer and more effective pharmaceuticals for consumers. For more information on pricing and to explore our full range of products, visit JM Science's website today!
Common Challenges and Misconceptions in Titration
Common challenges in the process often arise from misjudging the endpoint, improper mixing, and issues with equipment calibration. A prevalent misconception is the belief that the endpoint is synonymous with the equivalence point, which can lead to significant inaccuracies in results. Studies indicate that misconceptions about these critical concepts can persist, with a notable decrease in misunderstanding to just 9.4% after targeted educational interventions in argentometric analysis.
Expert insights reveal that many students struggle with the conceptual aspects of the process, often relying on algorithmic problem-solving rather than a deep understanding of the underlying principles. This dependence can obstruct their capacity to accurately interpret results and apply volumetric analysis techniques effectively. As Issa I. Salame observes, "Students face difficulties when studying concepts related to this technique and depend on algorithmic problem-solving and calculation methods to address issues associated with this subject instead of the topic."
This emphasizes the necessity for educational methods that not only tackle misconceptions but also promote a deeper understanding of the principles involved. To overcome these challenges, it is crucial to implement comprehensive training programs that emphasize both conceptual understanding and practical skills. Case studies, such as those discussed in the article "Conceptual vs. Algorithmic Teaching in Chemistry," highlight the effectiveness of teaching strategies that promote a multi-faceted understanding of chemical phenomena. These strategies motivate learners to understand the differences between macroscopic observations and particulate behaviors, ultimately resulting in more precise measurement practices.
Furthermore, advancements in technology, such as the LabX™ Titration software, enable the connection of scientific instruments, streamlining analysis processes through electronic data flow. This innovation not only improves flexibility but also guarantees adherence to strict testing criteria, further aiding precise and dependable measurement results. JM Science Inc.'s dedication to refreshing product selections and sustaining robust connections with producers guarantees that research facilities have access to the most current technologies and solutions, effectively tackling the prevalent challenges and misunderstandings in volumetric analysis practices.
By addressing these issues through targeted education and technological integration, laboratories can significantly enhance their measurement processes and results.
The Importance of Titrant and Analyte in Accurate Titration Results
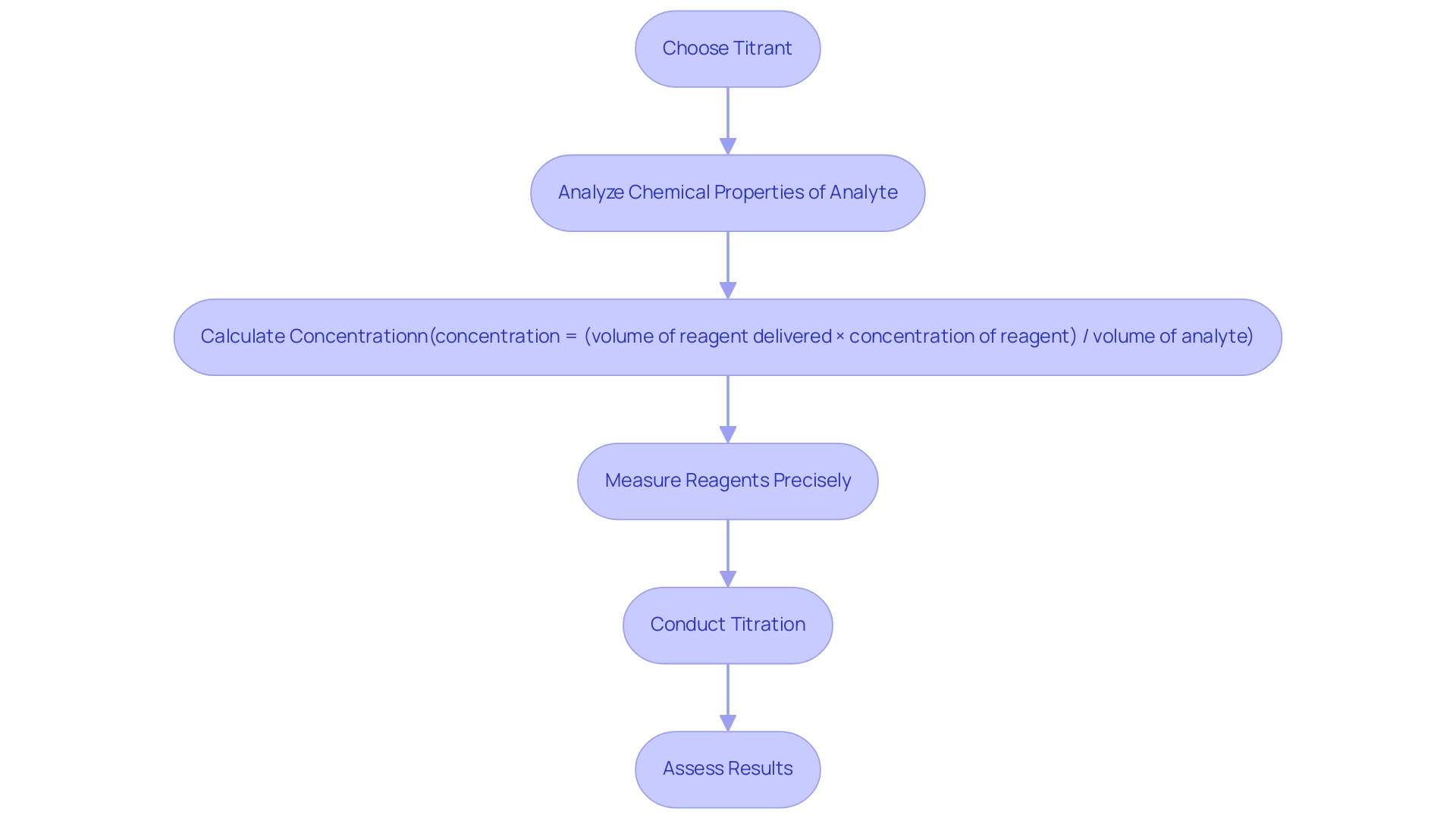
Understanding the roles of titrant versus analyte is essential for achieving accurate and reliable titration results. The choice of reagent must align carefully with the chemical properties of the analyte to ensure a complete and effective reaction. For instance, the concentration of the unknown solution can be calculated using the formula:
concentration = (volume of reagent delivered × concentration of reagent) / volume of analyte
This highlights the importance of precise measurement of the reagent.
Precise measurement results are crucial in laboratory practices, as they directly affect analytical outcomes. Practical examples illustrate how the connection between reagent and analyte influences results. In back analysis, for example, a known surplus of reagent is added to a solution, and the surplus is later analyzed. This method proves particularly useful for slow reactions or non-soluble solids, allowing for more accurate endpoint determination in complex analytical scenarios.
The case study titled "Back Titration Explained" showcases the utility of this method, demonstrating its effectiveness in various analytical contexts.
Data on measurement precision within this process underscores its impact on outcomes. Studies indicate that even minor discrepancies in titrant volume can lead to significant variations in calculated concentrations, emphasizing the need for meticulous measurement practices. Expert opinions bolster this notion, with many analytical chemists asserting that precise measurement is fundamental to the integrity of volumetric results.
As one expert noted, "Titration embodies the essence of analytical chemistry—precision, versatility, and depth of insight into chemical properties."
Furthermore, sustainability principles are being explored in measurement techniques to minimize environmental impact while maintaining analytical integrity. Researchers are investigating methods to reduce waste and enhance the efficiency of measurement processes. The use of primary standards to calibrate secondary standards enhances quality control in analytical processes, ensuring reliable results.
In conclusion, the careful selection of titrant versus analyte, along with rigorous measurement practices, is vital for achieving accurate titration results. By focusing on these elements, laboratory professionals can enhance their analytical techniques, contributing to more effective research and quality control processes.
Conclusion
Titration serves as a cornerstone of analytical chemistry, crucial for the precise determination of unknown solution concentrations. By delving into the fundamental principles of titrants and analytes, alongside various methodologies, the importance of mastering this technique becomes unmistakable. The distinction between the titrant—a solution of known concentration—and the analyte, which possesses an unknown concentration, is essential for achieving reliable results. Selecting titrants that align with the specific chemical properties of analytes is paramount for ensuring accurate reactions and valid endpoints.
This article underscores the practical applications of titration across various fields, particularly in pharmaceuticals and environmental testing. Titration not only adheres to stringent regulatory standards but also bolsters quality control processes vital for product consistency. Furthermore, advancements in titration technology, including automated systems and software integration, have streamlined laboratory practices, minimizing human error and enhancing efficiency.
Nonetheless, challenges and misconceptions surrounding titration persist, often stemming from misunderstandings of key concepts such as the equivalence point and endpoint. Addressing these issues through targeted education and technological support is crucial for cultivating a deeper comprehension of titration principles. Highlighting the significance of accurate measurement and method selection can substantially improve the reliability of analytical outcomes.
Ultimately, mastering titration techniques is indispensable for professionals committed to upholding the highest standards in analytical practices. By embracing the complexities of titration and leveraging innovative solutions, laboratories can drive advancements in research, quality control, and environmental safety, ensuring that accurate and reliable results remain at the forefront of their analytical endeavors.




