Overview
Analyzers in pharmaceutical labs serve as indispensable instruments, executing both quantitative and qualitative assessments of materials, including biological fluids. Their essential function is to guarantee the safety and quality of medicinal products. This article underscores their pivotal role in drug development and quality assurance processes, illustrating how advanced techniques and automation significantly enhance accuracy, efficiency, and compliance with regulatory standards. By integrating these sophisticated tools, laboratories can ensure that their outputs meet the highest standards of quality and safety, ultimately fostering trust in medicinal products.
Introduction
In the intricate world of pharmaceutical laboratories, analyzers stand as the backbone of quality control and drug development, transforming raw data into actionable insights. These sophisticated instruments not only measure chemical compositions but also ensure that medications adhere to stringent safety standards, playing a pivotal role in safeguarding patient health. As the demand for rapid and reliable testing methods escalates, the evolution of these tools to meet the challenges of modern science becomes imperative. Understanding the multifaceted functions and significance of analyzers is essential for anyone invested in the future of pharmaceutical innovation.
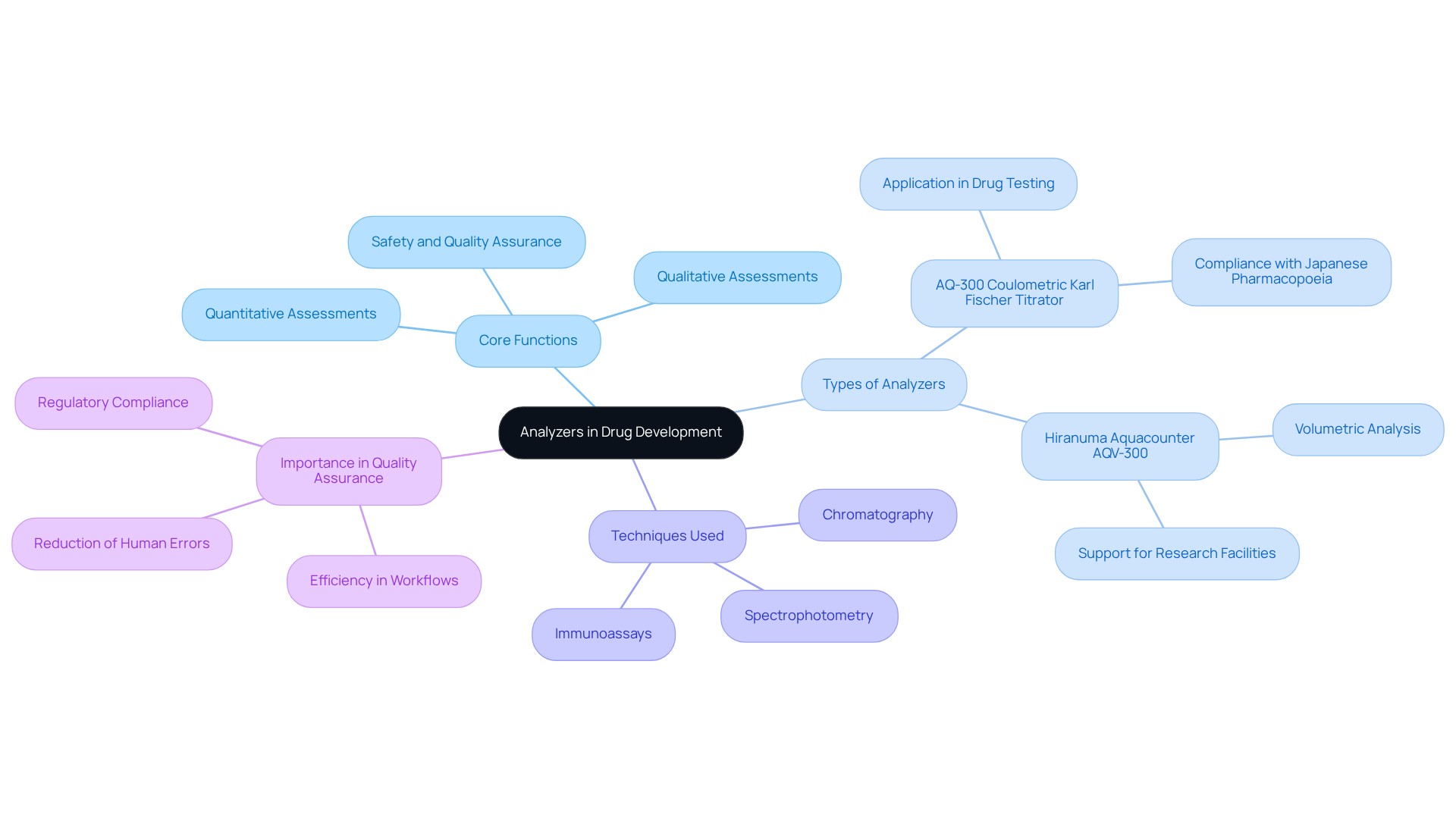
Define an Analyzer: Core Concepts and Functions
In laboratory settings, particularly within drug development facilities, understanding what is an analyzer is crucial for measuring and assessing the characteristics of different materials. These instruments are pivotal for conducting both quantitative and qualitative assessments of samples, including blood, urine, and other biological fluids, prompting the inquiry of what is an analyzer. By employing advanced techniques such as spectrophotometry, chromatography, and immunoassays, these instruments demonstrate what is an analyzer by providing accurate information on chemical compositions, concentrations, and other essential parameters. This functionality is vital for guaranteeing the standard and safety of medicinal products, as well as for facilitating research and development activities.
Among the advanced analyzers available, the AQ-300 Coulometric Karl Fischer Titrator stands out for its application in drug and medicine testing, particularly in compliance with the Japanese Pharmacopoeia. This titrator is designed to precisely assess moisture levels in medications, ensuring that products adhere to stringent safety standards. Similarly, the Hiranuma Aquacounter AQV-300 Volumetric Karl Fischer Titrator offers reliable volumetric analysis, further supporting research facilities in their assurance processes.
The influence of assessment tools on quality assurance in pharmaceutical facilities is significant. They empower research facilities to uphold rigorous standards by providing precise and dependable measurements, which are essential for compliance with regulatory requirements. Furthermore, the integration of these instruments enhances efficiency in the workspace by optimizing workflows and reducing the likelihood of human errors. As emphasized by industry specialists, the importance of these tools in assessing material characteristics is crucial for advancing drug research and ensuring patient safety. Ultimately, assessors play a crucial role in in testing operations, fostering both innovation and assurance within the drug industry.
Contextualize the Role of Analyzers in Pharmaceutical Laboratories
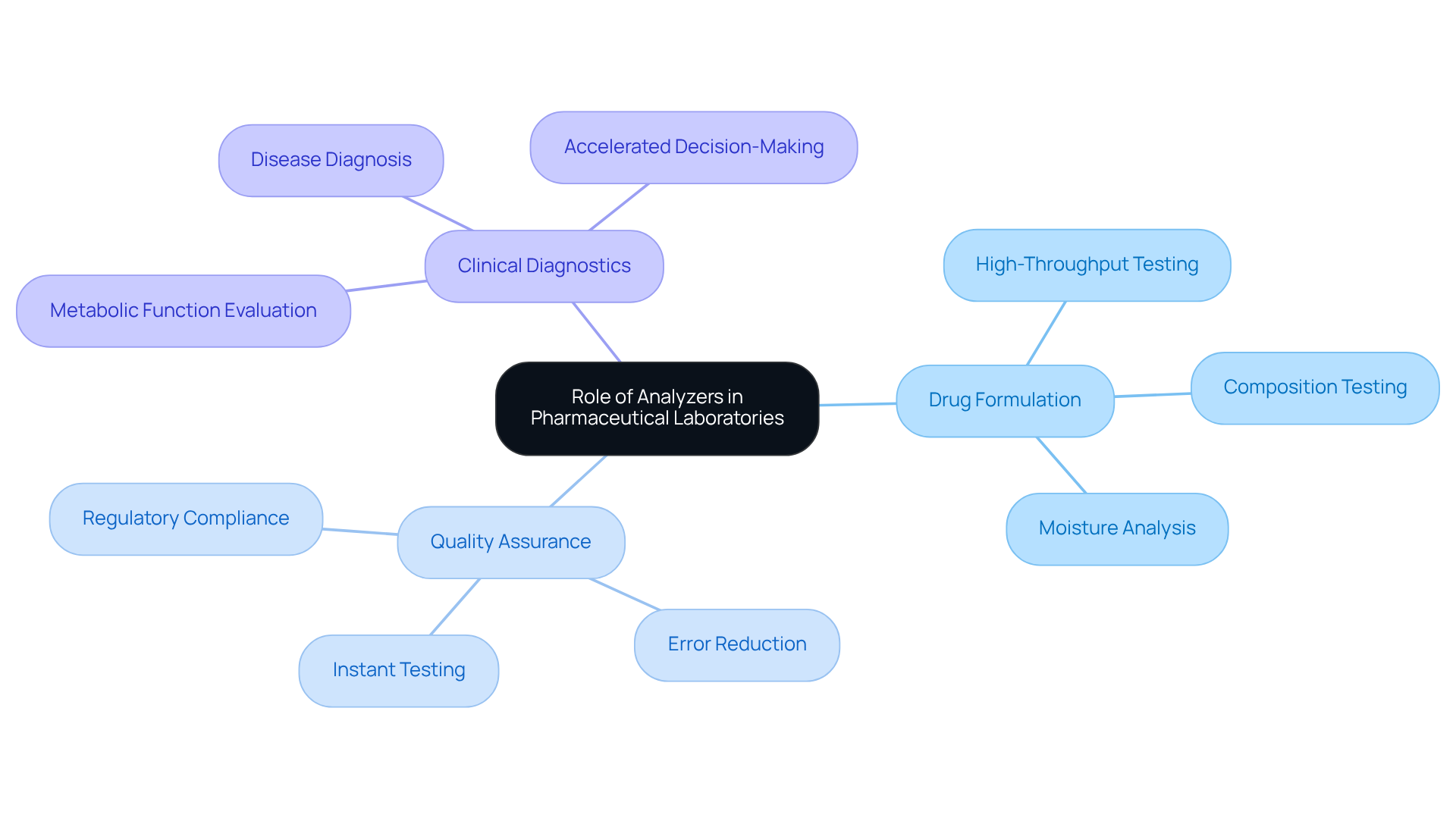
In pharmaceutical facilities, testing devices serve as essential tools that facilitate a range of critical tasks, including drug formulation, quality assurance, and clinical diagnostics. These devices enable , which is indispensable for complying with regulatory standards and ensuring patient safety.
For instance, blood chemistry testing devices are pivotal in evaluating metabolic functions and diagnosing diseases, whereas composition testing equipment confirms that drug formulations meet established specifications.
The integration of assessment tools into laboratory workflows significantly enhances efficiency, reduces human error, and accelerates the testing process, thereby enabling timely decision-making in drug development and improving patient care.
As one lab manager noted, the introduction of advanced instruments has transformed our diagnostic capabilities, allowing us to deliver faster and more accurate results—an essential requirement in today's fast-paced pharmaceutical environment.
Trace the Evolution of Analyzers in Scientific Research
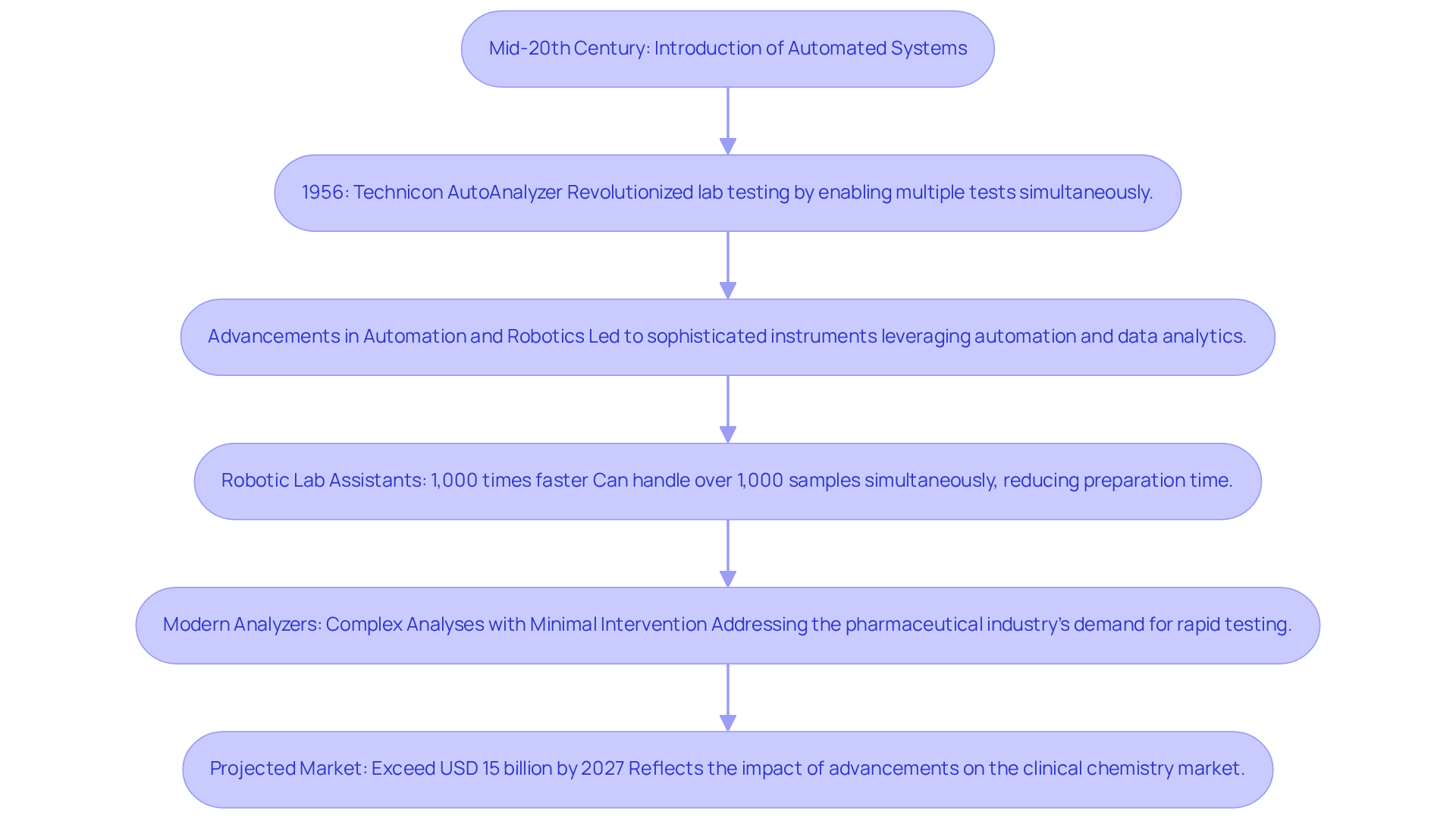
The development of measurement tools can be traced back to the mid-20th century, marking the introduction of the first automated systems. The Technicon AutoAnalyzer, developed by Leonard Skeggs in 1956, revolutionized laboratory testing by enabling multiple tests to be conducted simultaneously. Over the decades, advancements in technology have led to the creation of increasingly sophisticated instruments that leverage automation, robotics, and data analytics. A study from the University of Liverpool illustrates this progress, revealing that robotic lab assistants can operate approximately 1,000 times faster than human technicians. This capability allows for the handling of over 1,000 samples simultaneously, significantly reducing preparation time and human error.
Understanding what is an analyzer is essential, as are now equipped to perform complex analyses with minimal human intervention, a crucial factor in addressing the pharmaceutical industry's growing demand for rapid and reliable testing methods. This evolution is particularly significant in light of stringent regulatory requirements that necessitate high accuracy and throughput in test results. The introduction of automated systems has not only enhanced operational efficiency but has also paved the way for innovations such as flow chemistry, which facilitates high-throughput experimentation.
As the landscape of laboratory technology continues to evolve, the emphasis on automation and advanced analytical capabilities remains paramount. The historical transition from manual testing to highly automated systems underscores the strategic importance of these instruments in enhancing diagnostic precision and supporting the growth of the drug industry. Furthermore, the global market for clinical chemistry instruments is projected to exceed USD 15 billion by 2027, reflecting the substantial impact of these advancements on the industry.
Identify Key Characteristics and Types of Analyzers
In pharmaceutical labs, an analyzer can be defined as essential tools that are categorized based on their specific functions and applications. Understanding these categories is crucial for effective laboratory operations. The primary types include:
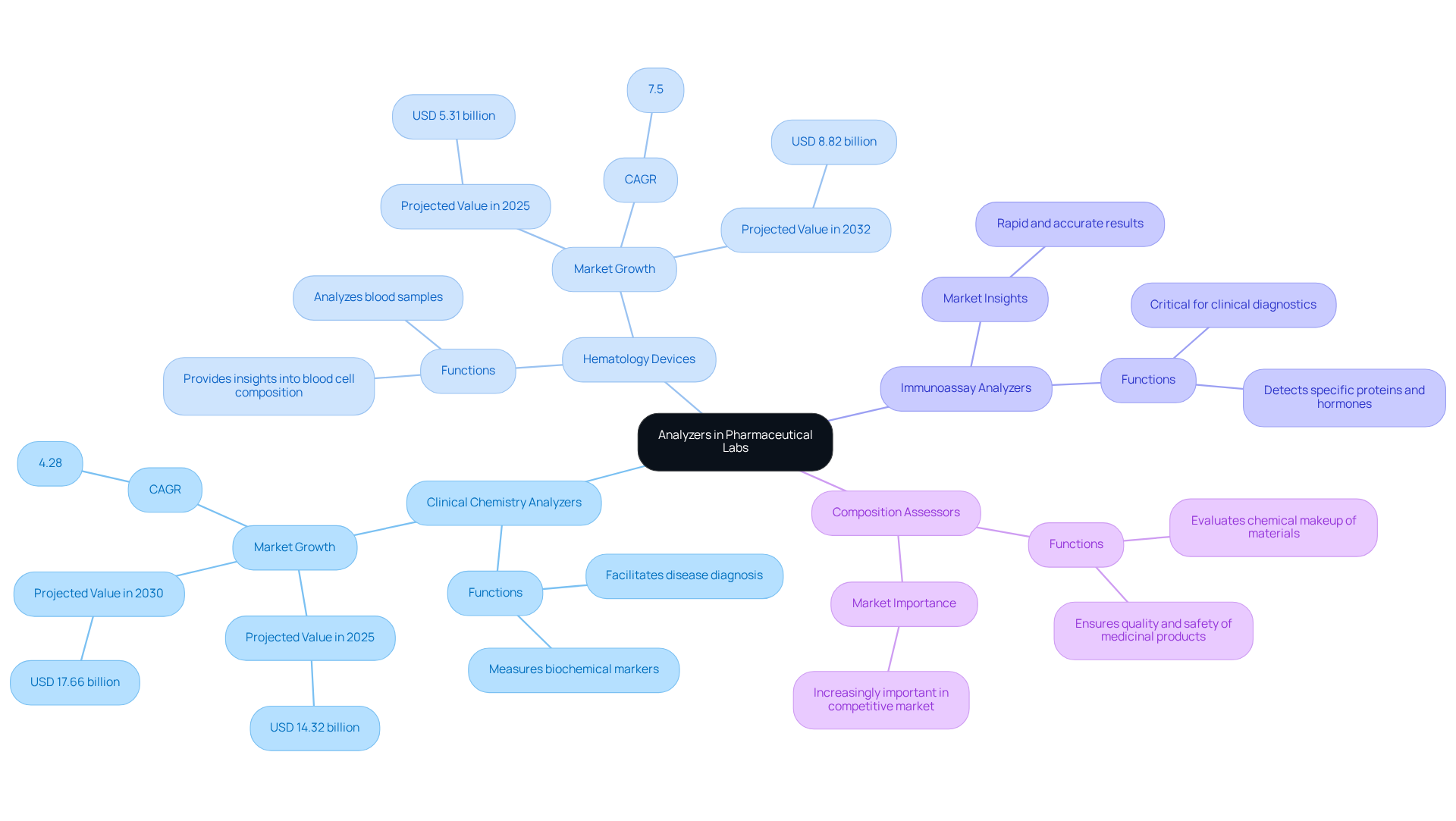
- Clinical Chemistry Analyzers: These instruments are vital for measuring various biochemical markers in blood and other bodily fluids, facilitating disease diagnosis and ongoing monitoring. The clinical chemistry testing equipment market is projected to expand from USD 14.32 billion in 2025 to USD 17.66 billion by 2030, reflecting a compound annual growth rate (CAGR) of 4.28%. This growth is primarily driven by the and the rising demand for accurate diagnostic tools.
- Hematology Devices: Specifically designed to analyze blood samples, hematology instruments provide detailed insights into blood cell composition and function. The hematology testing market is anticipated to grow significantly, with a forecasted value of USD 5.31 billion in 2025, reaching USD 8.82 billion by 2032. This growth is propelled by the increasing prevalence of blood disorders and an aging demographic.
- Immunoassay Analyzers: These devices are essential for detecting and quantifying specific proteins, hormones, or drugs, playing a critical role in clinical diagnostics. Their ability to deliver rapid and accurate results is crucial for effective patient management.
- Composition Assessors: Employed to evaluate the chemical makeup of various materials, these devices ensure the quality and safety of medicinal products, which is increasingly important in a competitive market.
Key characteristics of effective analyzers include:
- Accuracy and Precision: These are essential for reliable test results, ensuring that diagnoses are based on correct data.
- Automation Capabilities: Automation enhances efficiency, enabling labs to process a higher volume of samples with minimal human intervention.
- High Sample Throughput: The ability to handle large numbers of samples quickly is crucial in busy pharmaceutical labs, where timely results can significantly impact patient care.
Choosing an analyzer usually depends on specific testing requirements and the nature of the samples being analyzed. As the demand for advanced diagnostic solutions continues to rise, the evolution of these instruments is expected to disrupt traditional approaches in healthcare, ultimately enhancing the overall quality of patient care.
Conclusion
Understanding the role of analyzers in pharmaceutical laboratories is crucial for maintaining the integrity and safety of medicinal products. These sophisticated instruments facilitate precise measurements and assessments of various materials, playing a pivotal role in ensuring compliance with stringent regulatory standards. The significance of analyzers extends beyond mere functionality; they are integral to advancing drug development and enhancing patient safety.
Throughout this discussion, insights into the types of analyzers—ranging from clinical chemistry analyzers to immunoassay devices—highlight their specific applications in drug formulation, quality assurance, and diagnostics. The evolution of these tools from manual systems to highly automated technologies underscores their growing importance in the pharmaceutical landscape. As the market for clinical chemistry instruments continues to expand, the demand for accurate and efficient testing solutions becomes increasingly critical.
In light of these developments, it is evident that the integration of advanced analyzers in pharmaceutical labs streamlines workflows and fosters innovation in drug research and development. The ongoing evolution of these instruments is a testament to their indispensable role in ensuring the quality and safety of pharmaceuticals. Embracing these advancements will ultimately lead to improved healthcare outcomes and a more efficient drug development process, reinforcing the importance of understanding what analyzers are and how they function within the industry.




