Overview
The article emphasizes the critical aspects of selecting and maintaining pH measuring devices, highlighting their significance in various scientific applications. It delineates essential criteria for device selection, including:
- Accuracy
- Calibration frequency
- Electrode care
Furthermore, it details maintenance practices that ensure reliable and precise pH measurements. This underscores the vital role these devices play in maintaining product quality and safety across multiple industries. By understanding these elements, professionals can enhance their laboratory practices and ensure optimal outcomes.
Introduction
Understanding pH measurement is crucial across various scientific fields, where the balance of acidity and alkalinity can significantly influence outcomes. This guide delves into the intricacies of selecting and maintaining pH measuring devices, offering insights that enhance accuracy and reliability in laboratory settings. With a plethora of devices available, one must consider how to determine the best choice for specific applications, ensuring optimal performance over time.
Understand pH Measurement Basics
pH quantifies the acidity or alkalinity of a solution on a scale from 0 to 14, with 7 representing neutrality. Values below 7 indicate acidity, while those above signify alkalinity. Understanding pH is essential across disciplines such as chemistry, biology, and environmental science, as it significantly impacts chemical reactions, biological functions, and product quality. The definition of pH as the negative logarithm of hydrogen ion concentration underscores its foundational role in scientific analysis. This understanding is crucial for selecting the appropriate pH measuring device and for comprehending its operational principles.
Recent advancements in pH gauging technology, including automatic temperature compensation and digital displays, have improved precision and convenience. It is essential for laboratories to remain informed about these innovations. For instance, in the food industry, precise pH measurement ensures the safety and quality of products, particularly dairy, where deviations can lead to spoilage or health risks. Moreover, data show that routine adjustment of pH devices is essential to uphold precision, with recommendations suggesting adjustment at least twice a week for optimal performance.
To ensure precise adjustment, it is important to use two pH standard buffer solutions (4.01 and 7.0) for laboratory-grade meters. Calibration should not be necessary more frequently than about once a month in typical general-purpose applications. By acknowledging the significance of pH assessment, laboratories can guarantee dependable outcomes that enhance the overall quality of their products.
Explore Types of pH Measuring Devices
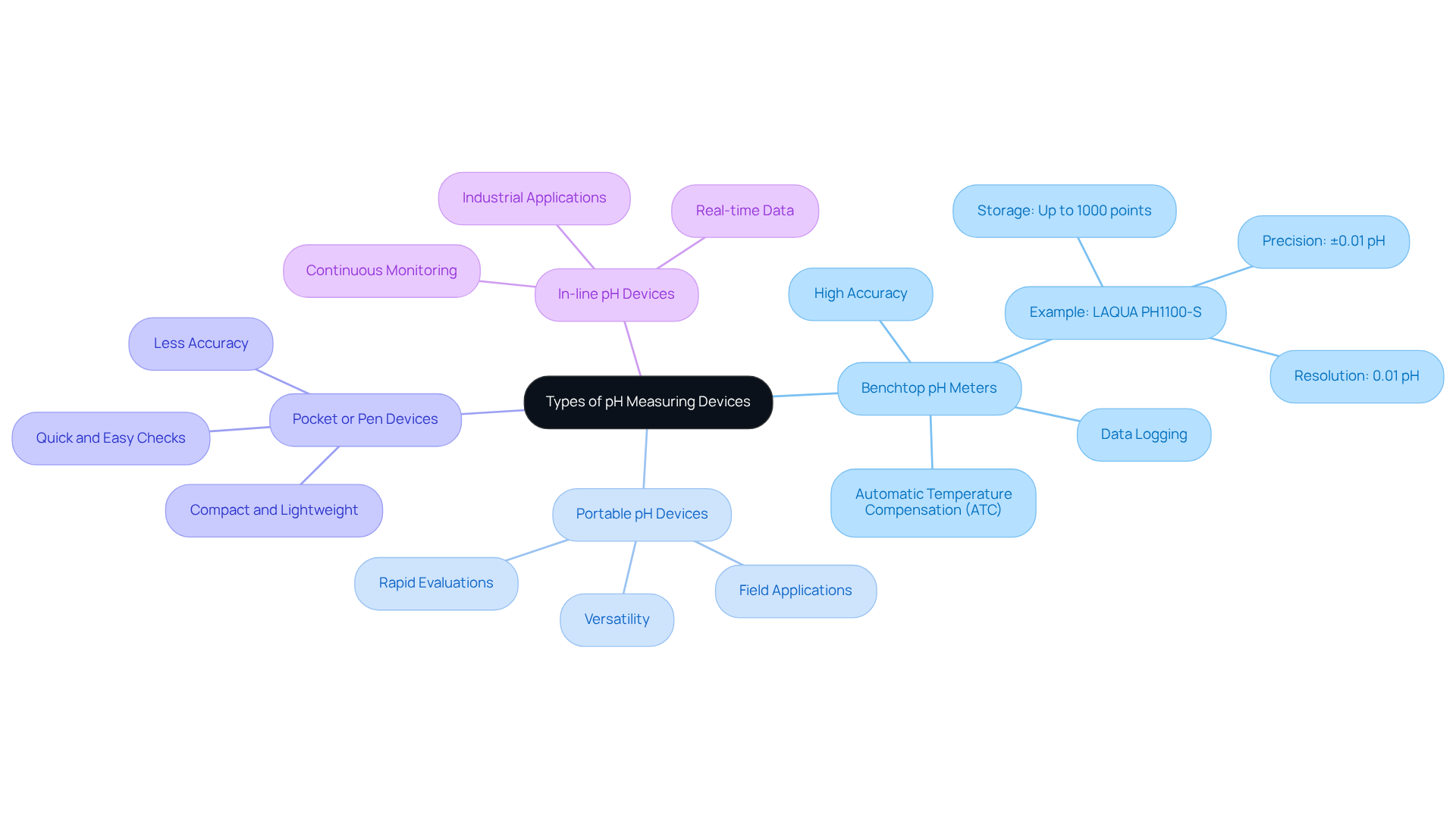
Several types of pH measuring devices are designed to cater to different needs and applications.
- Benchtop pH Meters are stationary devices renowned for their high accuracy, making them ideal for laboratory environments. They often feature advanced capabilities such as automatic temperature compensation (ATC) and data logging, which enhance their usability in precise measurements. For instance, the LAQUA PH1100-S device boasts a resolution of 0.01 pH and a precision of ±0.01 pH, establishing it as a dependable option for laboratory settings.
- Portable pH Devices are designed for field applications, striking a balance between portability and accuracy. These versatile tools are suitable for environmental monitoring, food testing, and other on-site measurements, allowing flexibility in various settings. Experts emphasize that the pH measuring device is essential for rapid evaluations, thereby improving efficiency and quality assurance in diverse environments.
- Pocket or Pen Devices are compact and lightweight, crafted for quick and easy pH checks. While they offer convenience, they may sacrifice some accuracy compared to more sophisticated models. These devices are best suited for quick evaluations rather than thorough analysis, making them ideal for on-the-go measurements.
- In-line pH Devices are integral to industrial processes, providing continuous, real-time monitoring of pH levels. This capability is essential for maintaining product quality and optimizing process efficiency in applications such as chemical manufacturing and wastewater treatment. Real-world applications demonstrate that these instruments are vital for ensuring adherence to quality management standards.
Grasping the differences between these instruments is crucial for selecting the most suitable pH measuring device for your specific application, ensuring precise and dependable readings. Furthermore, it is essential to calibrate pH devices prior to use, as calibration with recognized buffer solutions is vital for preserving measurement integrity.
Evaluate Key Selection Criteria for pH Meters
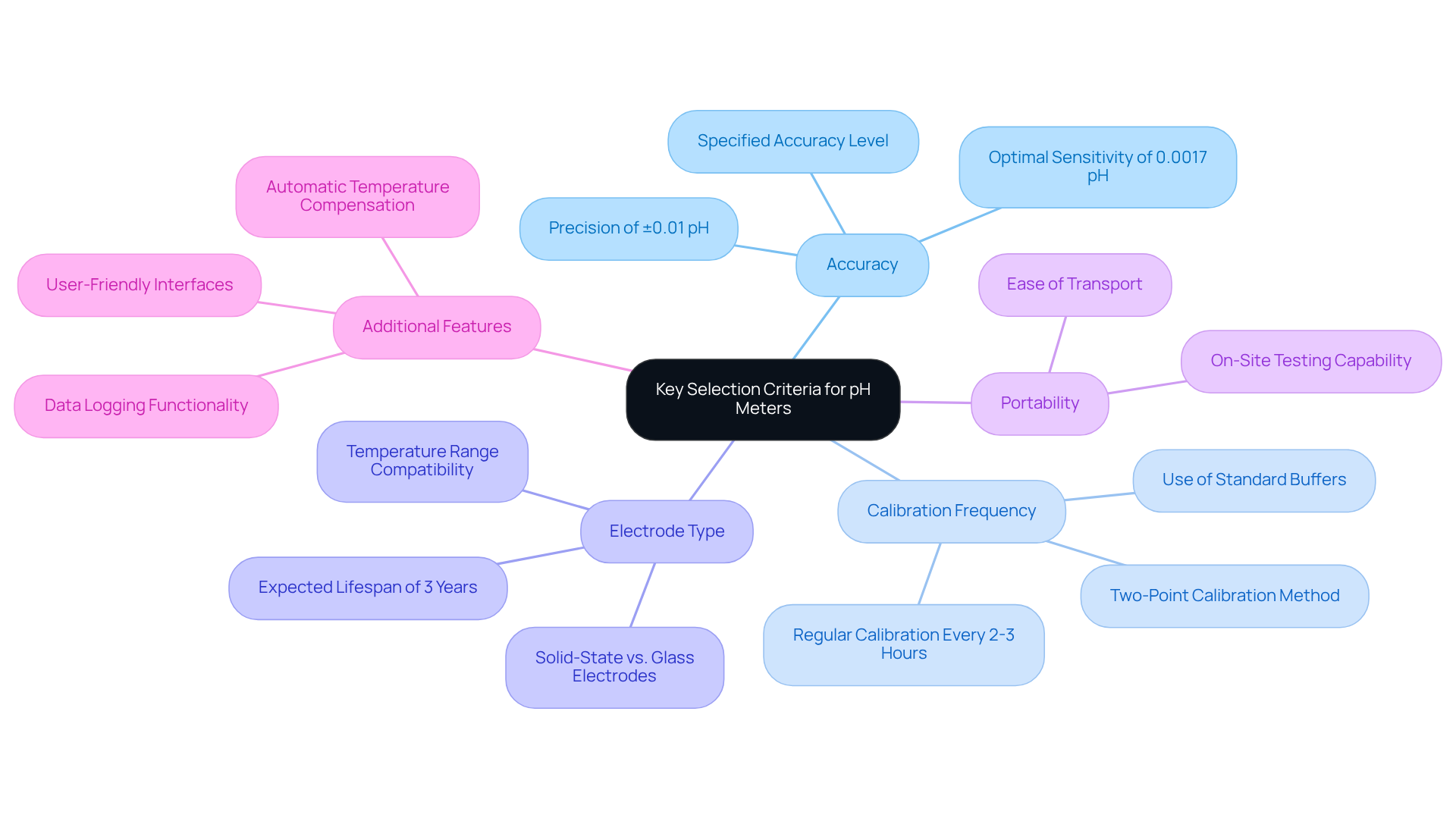
When selecting a pH meter for laboratory applications, it is crucial to consider several key criteria that ensure optimal performance and accuracy.
- Accuracy is paramount; choose meters with a specified accuracy level that aligns with your application needs. For laboratory work, it is often essential to use a pH measuring device with a precision of ±0.01 pH, as even minor deviations can significantly impact results. The optimal sensitivity of a pH device is determined to be 0.0017 pH, establishing a standard for acceptable precision.
- Next, consider Calibration Frequency. Regular calibration is essential for ensuring accuracy in readings. It is advised to calibrate pH devices at least every 2-3 hours, particularly when assessing different sample types or under varying environmental conditions. Calibration should be performed using at least two points close to the expected pH of the sample solution to ensure reliability. This practice reduces variation in assessment, ensuring dependable results.
- The Electrode Type is another critical factor. Different electrodes cater to various applications, so assess factors such as temperature range, sample compatibility, and maintenance requirements. For instance, solid-state pH electrodes may offer improved reliability compared to traditional glass electrodes. Additionally, a pH electrode is expected to last approximately 3 years under normal conditions, which is an important consideration for maintenance and replacement.
- If field evaluations are necessary, Portability becomes a significant consideration. Choose a portable device that is easy to transport and operate, particularly for applications requiring on-site testing.
- Lastly, explore Additional Features. Look for enhancements like automatic temperature compensation, data logging, and user-friendly interfaces. These features can significantly improve usability and efficiency in laboratory settings. Moreover, utilizing standard buffers for adjustment is crucial to guarantee precise pH readings, as emphasized by measurement specialists.
By thoroughly assessing these criteria, you can make a knowledgeable choice when acquiring a pH device, ensuring it satisfies the particular requirements of your laboratory applications.
Implement Maintenance and Calibration Practices
To ensure effective maintenance and calibration of your pH meter, it is imperative to adhere to the following best practices:
- Regular Calibration: It is essential to calibrate your pH meter before each use or at least weekly, depending on its frequency of utilization. Employ high-quality buffer solutions, particularly pH 4.0, 7.0, and 10.0, to guarantee precise adjustments. Routine calibration is crucial; research indicates that incorrect settings can lead to significant errors in pH assessments, adversely affecting laboratory outcomes. Furthermore, intelligent digital sensors can automate pH assessment processes and provide real-time diagnostics, thereby enhancing calibration precision.
- Electrode Care: After each use, rinse the electrode with distilled water and store it in an appropriate storage solution to maintain hydration. It is vital to avoid allowing the electrode to dry out, as this can result in irreversible damage and sluggish response times. Regular inspection of electrodes is essential; laboratory managers emphasize that neglecting electrode care can lead to drifting readings and compromised accuracy. As one laboratory manager stated, "Proper electrode maintenance is vital for ensuring consistent and reliable pH measurements."
- Cleaning: Routinely clean the electrode with suitable cleaning solutions to remove residues that may interfere with performance. Adhere to the manufacturer's cleaning guidelines to ensure optimal functionality.
- Storage: When not in use, it is important to keep the pH device in a secure, dry location. Proper storage of the electrode is critical to prevent damage and maintain its sensitivity.
- Troubleshooting: Should you encounter issues with readings, check the electrode for damage, recalibrate the device, and confirm that it is functioning properly. Frequent problems, such as erratic readings, can often be traced back to electrode maintenance issues or inadequate adjustment methods. For example, erratic readings may stem from a dirty electrode or unstable connections.
- Pricing Information: Consider the cost implications of maintaining your pH devices, including the pricing of adjustment solutions, which are available for approximately $20.74 for pH 4.00, 7.00, and 10.00 solutions.
- Temperature Compensation: Utilizing pH meters equipped with automatic temperature compensation (ATC) can significantly enhance calibration precision, ensuring that temperature fluctuations do not affect your measurements.
By implementing these practices, you will enhance the accuracy and reliability of your pH measuring device, ensuring it satisfies the rigorous demands of laboratory environments.
Conclusion
Understanding the intricacies of pH measurement is fundamental for achieving accuracy and reliability in various scientific and industrial applications. Mastering the selection and maintenance of pH measuring devices ensures that the measurements taken are precise and relevant to the specific needs of the task at hand. This knowledge enhances product quality and supports compliance with safety and quality standards across industries.
The article underscores the importance of grasping the basics of pH measurement, exploring different types of pH devices, evaluating selection criteria, and implementing effective maintenance practices. Key insights include:
- The significance of regular calibration
- The necessity of appropriate electrode care
- The advantages of selecting devices tailored to specific applications
Understanding these elements empowers users to make informed decisions, ultimately leading to improved outcomes in their respective fields.
In conclusion, meticulous attention to pH measurement practices profoundly impacts scientific analysis and product quality. As industries increasingly rely on precise pH readings, adopting best practices for device selection and maintenance becomes crucial. Embracing advancements in pH measurement technology and committing to regular calibration and care will enhance measurement accuracy and foster a culture of quality and reliability in all scientific endeavors.




