Overview
This article offers a comprehensive step-by-step guide on calibrating a pH meter, underscoring the necessity of regular calibration for precise pH measurements. It delineates essential practices, including:
- The use of fresh buffer solutions
- An understanding of calibration techniques—both single-point and multi-point
Furthermore, it emphasizes the importance of maintaining the pH meter to ensure reliable results. Ultimately, this highlights how proper calibration is vital for scientific accuracy and compliance within laboratory environments.
Introduction
In the realm of scientific research and industrial applications, the precision of pH measurements is paramount. pH meters—sophisticated instruments designed to gauge the acidity or alkalinity of solutions—serve as essential tools across various disciplines, from environmental monitoring to pharmaceuticals. Understanding their functionality, calibration techniques, and maintenance practices is crucial for laboratory professionals aiming to achieve reliable results.
As technology advances, the importance of accurate pH measurements becomes even more pronounced, underscoring the need for effective calibration methods and the selection of appropriate buffers. This article delves into the intricacies of pH meters, offering insights into their operation, the significance of calibration, and best practices to ensure optimal performance in laboratory settings.
Understanding pH Meters: Basics and Functionality
A pH gauge is a sophisticated electronic instrument designed to measure the acidity or alkalinity of a solution. At its core, it features a pH-sensitive electrode that generates a voltage directly proportional to the hydrogen ion concentration in the solution. This voltage is then transformed into a pH reading, supplying crucial information for diverse uses in laboratory and industrial environments.
Understanding how to calibrate a pH meter is vital for successful calibration and upkeep. The glass electrode, sensitive to hydrogen ions, plays a pivotal role in the evaluation process. Complemented by a reference electrode that provides a stable reference voltage, this combination ensures accurate readings.
Together, these electrodes enable precise pH measurements, essential for preserving the integrity of experimental results.
Recent statistics indicate that approximately 80% of laboratories utilize , underscoring their importance in scientific research and quality control processes. Furthermore, advancements in pH measurement technology have led to increased automation in routine tasks, allowing for cost and time savings. Particularly in smaller labs, heightened competition has made more entry-level options accessible.
Real-world applications of pH devices span a wide range of fields, including environmental monitoring, pharmaceuticals, and food safety. In North America, the demand for pH devices is expected to grow significantly, driven by the need for water testing in treatment plants, influenced by industrialization and stringent environmental regulations. Government initiatives aimed at reducing water pollution further support this trend.
Significantly, the regional expansion of the pH gauge market in North America underscores the rising significance of precise readings in adherence to these regulations.
Expert views emphasize the significance of pH-sensitive electrodes in guaranteeing accuracy. As noted by Dr. Surat P., a Ph.D. in Cell Biology and Mechanobiology, understanding the factors affecting pH instrument accuracy is crucial for laboratory professionals. This knowledge enhances the reliability of results and contributes to the overall efficiency of laboratory operations.
Moreover, precise pH readings can greatly minimize slide errors in histology laboratories by as much as 90%, highlighting the vital importance of pH devices in attaining accurate laboratory results.
In conclusion, the effectiveness of pH devices in laboratory environments is supported by their design and the technology that powers them. As laboratories continue to evolve, staying informed about how to calibrate a pH meter, along with the latest advancements and best practices, will be essential for maintaining high standards of accuracy and reliability.
The Importance of pH Meter Calibration
Understanding how to calibrate a pH meter is a fundamental practice for pH devices, ensuring that readings align with established standards and thereby guaranteeing precision. In laboratory environments, it is advised to calibrate the pH meter at least once a day, particularly when utilized for critical applications. Neglecting routine adjustments can lead to substantial reading drift, frequently linked to factors such as electrode aging, contamination, and environmental variations.
Research indicates that the average absolute discrepancy for standard operating procedures (SU OPT) can reach 0.072 pH units, underscoring the potential for error without proper calibration. Neglecting this calibration has far-reaching consequences, impacting research outcomes, product quality, and adherence to industry regulations. A case study on reference electrode stability emphasizes the importance of maintaining , which are vital for precise pH evaluations. This study promotes the use of distinct containers for reference electrodes to ensure consistent conditions, thereby improving the reliability of results.
Additionally, K. L. Cheng suggests that a new type of reference electrode and the use of a conducting wire to replace the salt bridge can significantly enhance pH accuracy. The effects of stirring, as well as the presence of double or triple layers on potential evaluation, further illustrate the complexities involved in pH assessment. Stirring can lead to a more uniform distribution of ions, while stagnant conditions may result in localized concentration gradients that affect readings.
Routine adjustments are essential for understanding how to calibrate a pH meter, enhancing the reliability of readings while also extending the lifespan of the pH device by reducing hazards linked to prolonged inaccuracies. Specialists in the domain highlight that advancements in novel reference electrodes and creative adjustment methods can considerably improve the stability and precision of pH readings, ultimately resulting in more dependable research results and enhanced product quality. For instance, recent analyses of N-glycans from monoclonal antibodies (mAb) produced under different pH conditions indicated similar patterns of glycosylation, underscoring the critical role of precise pH control in research outcomes.
Types of pH Meter Calibration: Single-Point vs Multi-Point
Adjusting pH devices is crucial for guaranteeing precise readings in laboratory environments, particularly regarding the [calibration of pH meters](https://jmscience.com), which primarily entails two techniques: single-point and multi-point adjustment.
Single-point adjustment is a straightforward approach where the meter is set to , typically pH 7. This method is quick and efficient, making it suitable for applications where the pH range remains stable. However, it has limitations, as it may not adequately address variations across a broader pH spectrum, potentially leading to inaccuracies in measurements.
As Colin Towers, an Element Trainer, observes, "Here we can see that zero fails within the 95% confidence limit values, and we can therefore say there is no significant difference between the given value of 0.0039 and zero, so a single point adjustment is justified." This emphasizes the context in which single-point adjustment may be acceptable, yet it underscores the need for caution in its application.
In contrast, multi-point adjustment refers to the calibration of pH meters by employing two or more buffer solutions—commonly pH 4, 7, and 10—to establish a more precise adjustment curve. This method is particularly advantageous for applications demanding high precision, as it effectively compensates for non-linearities in the electrode response across different pH levels. Studies show that laboratories employing significantly enhanced precision, with a considerable percentage of facilities embracing this method to improve their reliability in assessments.
For instance, a proposed real-time blood pressure measurement system demonstrated a mean accuracy of 98.22% for systolic blood pressure and 95.58% for diastolic blood pressure, illustrating the critical nature of precision in adjustment.
Expert opinions emphasize the calibration of pH meters, highlighting the significance of multi-point adjustment in achieving consistent results. Industry leaders advocate for this method, especially in environments where pH levels fluctuate frequently. A comparative analysis of adjustment methods reveals that multi-point adjustment not only enhances accuracy but also aligns with current best practices in the field.
Real-world examples further demonstrate the effectiveness of multi-point adjustment. In various laboratory settings, implementing this technique has led to improved data integrity and confidence in results, particularly in critical applications such as pharmaceutical testing and environmental monitoring. A pertinent case study on a Continuous Blood Pressure Monitoring Platform illustrates the importance of precise data collection, highlighting the relevance of multi-point adjustment in laboratory environments.
By implementing multi-point adjustments, laboratories can ensure that their pH measurements are both reliable and precise, which is crucial for understanding the calibration of pH meters, ultimately supporting better decision-making in scientific research.
JM Science Inc. is dedicated to supplying high-quality instruments and support resources that enhance these measurement techniques, reinforcing the credibility of the methods discussed and linking them to the company's innovative offerings.
Step-by-Step Guide to Calibrating Your pH Meter
- Prepare Calibration Solutions: Begin by gathering at least two fresh and uncontaminated buffer solutions, typically pH 4.00 and pH 7.00. These solutions are essential for precise adjustment and should not be reused or returned to their original containers to prevent contamination. The cost of these adjustment solutions is approximately 20.74 USD.
Turn on : Power on the pH meter and allow it to warm up for a few minutes, as this can enhance measurement stability and accuracy. - Rinse the Electrode: Thoroughly rinse the pH electrode with distilled water to eliminate any contaminants that could impact the adjustment process.
Immerse in Buffer Solution: Submerge the electrode in the first buffer solution (e.g., pH 7.00) and wait for the reading to stabilize. This step is crucial for obtaining an accurate measurement point.
Set Reference Point: Follow the manufacturer's instructions to establish the reference point for pH 7.00. This establishes a reference for subsequent measurements. - Repeat for Additional Solutions: After rinsing the electrode again, immerse it in the second solution (e.g., pH 4.00) and allow the reading to stabilize. Set this as the second adjustment point.
Verify Calibration: To ensure accuracy, test the calibrated meter with a third buffer solution, ideally pH 10.00. If discrepancies occur, make necessary adjustments to the tuning settings.
Document Adjustment: Finally, record the adjustment date and results in a log for future reference. This documentation is crucial for upholding compliance and guaranteeing continuous precision in measurements.
Regular adjustment of pH devices is necessary for preserving measurement accuracy, standardization, and safety in laboratory settings. The typical duration for adjustment usually varies from 10 to 15 minutes, based on the intricacy of the device and the quantity of adjustment points needed. A case study on microprocessor-based pH devices emphasizes their benefits, such as simplicity of use and reduced handling mistakes through a menu-guided adjustment process.
By adhering to these best practices, laboratories can significantly reduce equipment downtime and enhance the reliability of their analytical results. If you are still uncertain how to calibrate a pH meter accurately or do not know which pH values of the solution best suit your needs, do not hesitate to contact the world-class team at Atlas Scientific.
Choosing the Right Calibration Solutions and Buffers
Choosing the appropriate adjustment options is crucial for understanding how to calibrate a [pH meter](https://jmscience.com) to ensure precise pH readings. Selecting solutions that align with the expected pH range of your samples is essential when learning how to calibrate a pH meter. The most frequently utilized solutions in laboratories comprise:
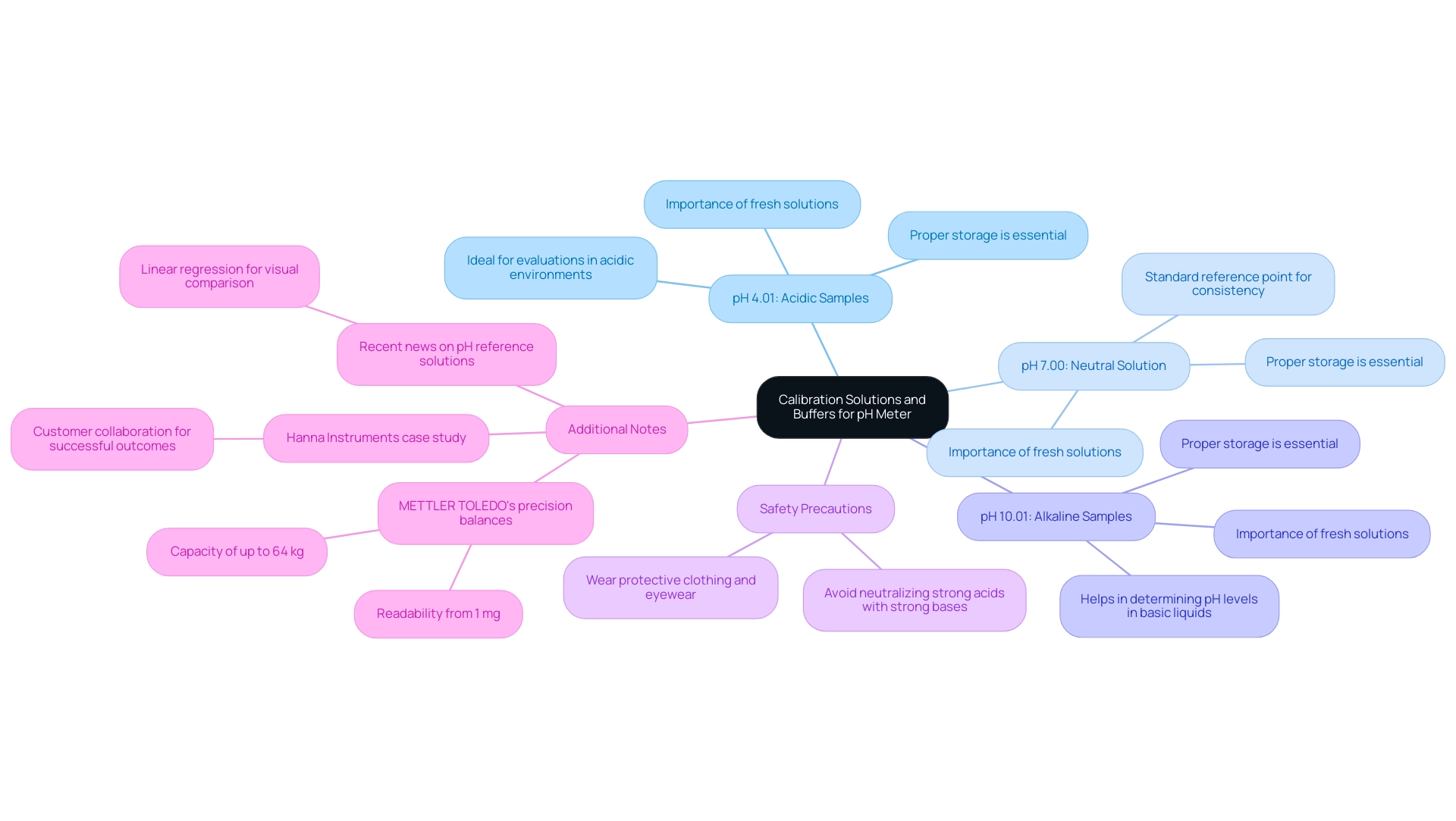
- pH 4.01: This solution is ideal for acidic samples, providing a dependable reference point for evaluations in acidic environments.
- pH 7.00: Known as , it acts as a standard reference point for adjusting pH meters, ensuring consistency across various applications.
- pH 10.01: Appropriate for alkaline samples, this solution aids in precisely determining pH levels in basic liquids.
To achieve optimal accuracy, it is essential that these solutions are fresh, properly stored, and traceable to recognized standards, such as those established by the National Institute of Standards and Technology (NIST). Utilizing outdated or tainted solutions can result in considerable adjustment mistakes, undermining the dependability of your readings.
In practice, the precision and trustworthiness of solution mixtures are crucial. Research has demonstrated that utilizing fresh standard solutions can improve precision, with some producers stating that their solutions retain a high level of accuracy even after prolonged use. Expert chemists highlight the importance of using fresh standard solutions, noting that the integrity of the buffer directly affects the adjustment process.
When choosing adjustment buffers, consider the specific needs of your laboratory's applications. This meticulous selection process not only guarantees precise pH readings but also demonstrates how to calibrate a pH meter to enhance the overall quality of your analytical outcomes.
Safety Note: Always wear protective attire and eyewear when dealing with acids and bases to ensure safety in laboratory environments.
Additionally, it is worth mentioning that METTLER TOLEDO's precision balances, which have a capacity of up to 64 kg and readability from 1 mg, can significantly improve accuracy in conjunction with proper adjustment practices. A case study from Hanna Instruments demonstrates the significance of customer cooperation in attaining successful testing results, emphasizing the necessity of choosing the appropriate adjustment solutions. Furthermore, as noted in recent news, the average of the normalized measurement results of secondary DKD pH reference solutions was used to calculate a linear regression for visual comparison with commercial solutions, emphasizing the significance of solution accuracy and reliability.
For more assistance in choosing measuring equipment, consider contacting your local representative for a complimentary balance recommendation, as suggested by METTLER TOLEDO.
Troubleshooting Common Calibration Issues
Frequent adjustment problems with pH meters can significantly impact laboratory results. Understanding these prevalent issues and their solutions is crucial for maintaining accuracy in measurements.
Drifting Readings are a common concern, often indicating electrode contamination or damage. To address this, thoroughly rinse the electrode with distilled water and recalibrate using fresh measurement solutions.
Inconsistent Results frequently arise from utilizing . Laboratories should ensure that storage units are stocked with fresh solutions and verify that the electrode is clean and well-maintained to achieve reliable readings.
Slow Response Time of the electrode may signal a need for cleaning or conditioning. Soaking the electrode in a suitable solution, as recommended by the manufacturer, can restore its responsiveness.
Calibration Failure can be frustrating. If the meter fails to calibrate, confirm that the correct buffer solutions are in use and that the meter is functioning properly. Consulting the user manual for specific troubleshooting steps can provide additional guidance.
Statistics indicate that approximately 30% of laboratories encounter measurement failures, underscoring the significance of regular maintenance and appropriate adjustment methods. Notably, the decrease of RPI for contaminated standards from 128 to 96 highlights the impact of effective adjustment practices on laboratory efficiency.
Practical applications of a Failure Mode and Effects Analysis (FMEA) process can yield substantial advancements in laboratory safety and efficiency. This systematic approach is essential for identifying and addressing potential failure modes in adjustment processes, ensuring that critical measurement methods are consistently evaluated and improved.
Expert troubleshooting advice emphasizes the importance of regularly checking adjustment buffers for expiration dates and maintaining a clean electrode to prevent common adjustment issues. As noted by Miller et al., "An essential attribute for is consistency and stability for all elements in the measurement hierarchy, especially when employing a correction for non-commutability." By proactively addressing these challenges, laboratory managers can enhance the reliability of their pH measurements and ensure accurate results in their analyses.
JM Science Inc. is committed to supporting laboratory managers by continually enhancing its product offerings and fostering strong partnerships with leading manufacturers. This dedication ensures access to the finest tools and resources for effective measurement.
Maintaining Your pH Meter for Optimal Performance
To ensure optimal performance and longevity of your pH meter, adhere to the following best practices:
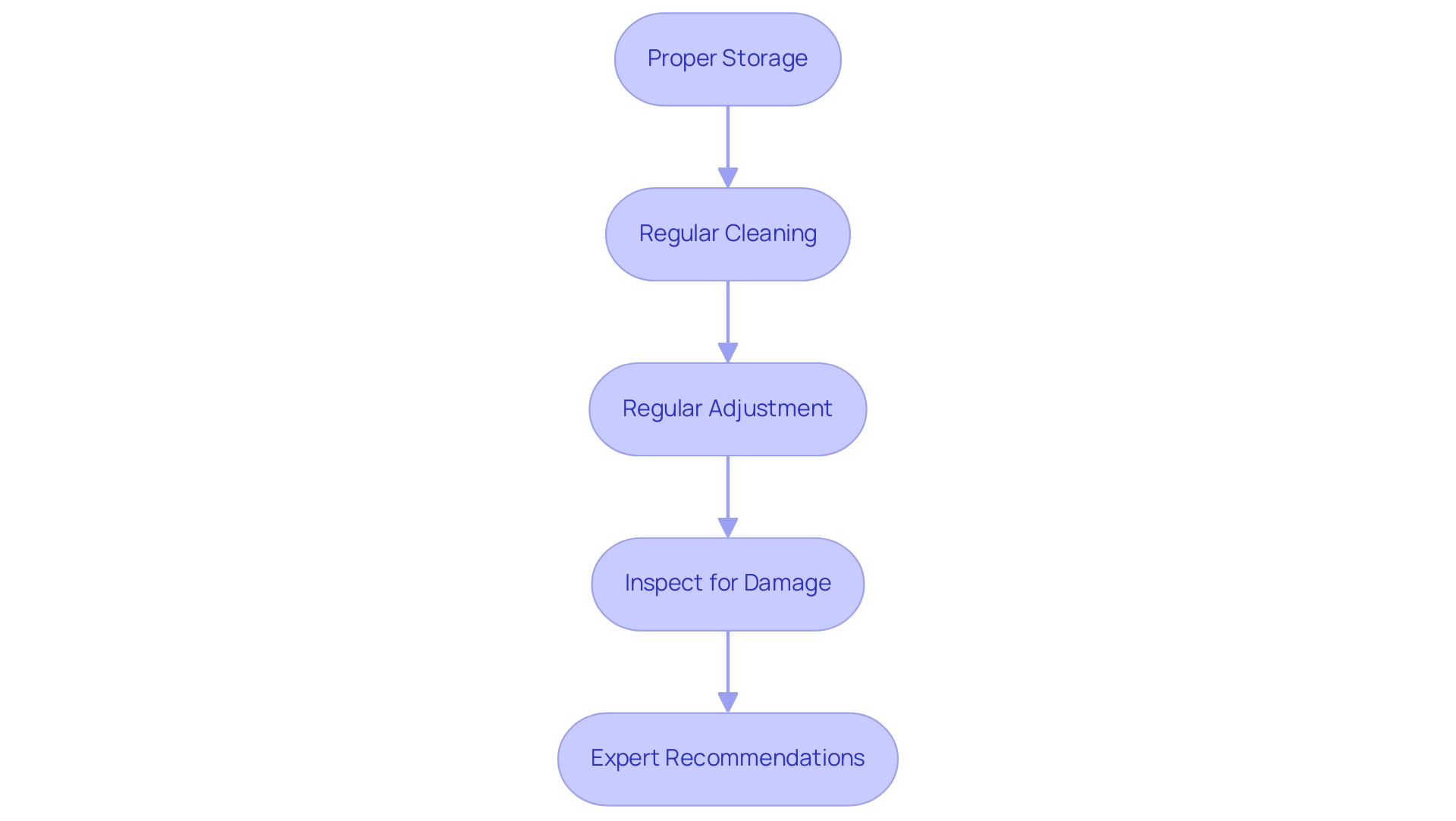
- Proper Storage: Always store the electrode in a suitable storage solution that maintains hydration. Avoid using distilled water for storage, as it can lead to electrode damage. Instead, opt for aqueous and ion-rich solutions to prolong the lifespan of the electrode.
- Regular Cleaning: Clean the electrode frequently using distilled water and a soft cloth. For more stubborn residues, a mild detergent solution can be effective. This routine cleaning helps uphold the precision of your measurements.
- Regular Adjustment: Understanding how to calibrate your pH meter is crucial, especially prior to performing important measurements. Adhere to the manufacturer's instructions regarding the frequency of adjustments to ensure precision. Utilizing certified solutions that meet standards such as DIN or NIST can enhance precision and traceability during adjustment. As emphasized in the case study on the significance of stabilizing solutions in pH meter adjustments, merging technical solutions for routine adjustments with certified solutions for critical applications achieves a balance between cost-effectiveness and precision.
- Inspect for Damage: Periodically check the electrode for any cracks or signs of wear. If any damage is detected, replace the electrode promptly to maintain accurate readings. The success narrative of Mitsubishi Chemical Corporation demonstrates the benefits of shifting from analog to digital pH sensors utilizing Intelligent Sensor Management (ISM) technology, which can further improve the reliability of readings.
- Expert Recommendations: Industry leaders stress the significance of verifying the expiry date of standard solutions prior to use to ensure dependable outcomes. Tejas Patel advises that this practice is essential for maintaining measurement integrity. Furthermore, merging technical buffers for regular adjustments with certified buffers for essential applications achieves a balance between cost-effectiveness and precision, as emphasized in case studies on .
By applying these strategies, you can greatly prolong the lifespan of your pH devices and guarantee consistent, accurate performance in your laboratory.
Calibration Frequency: How Often Should You Calibrate?
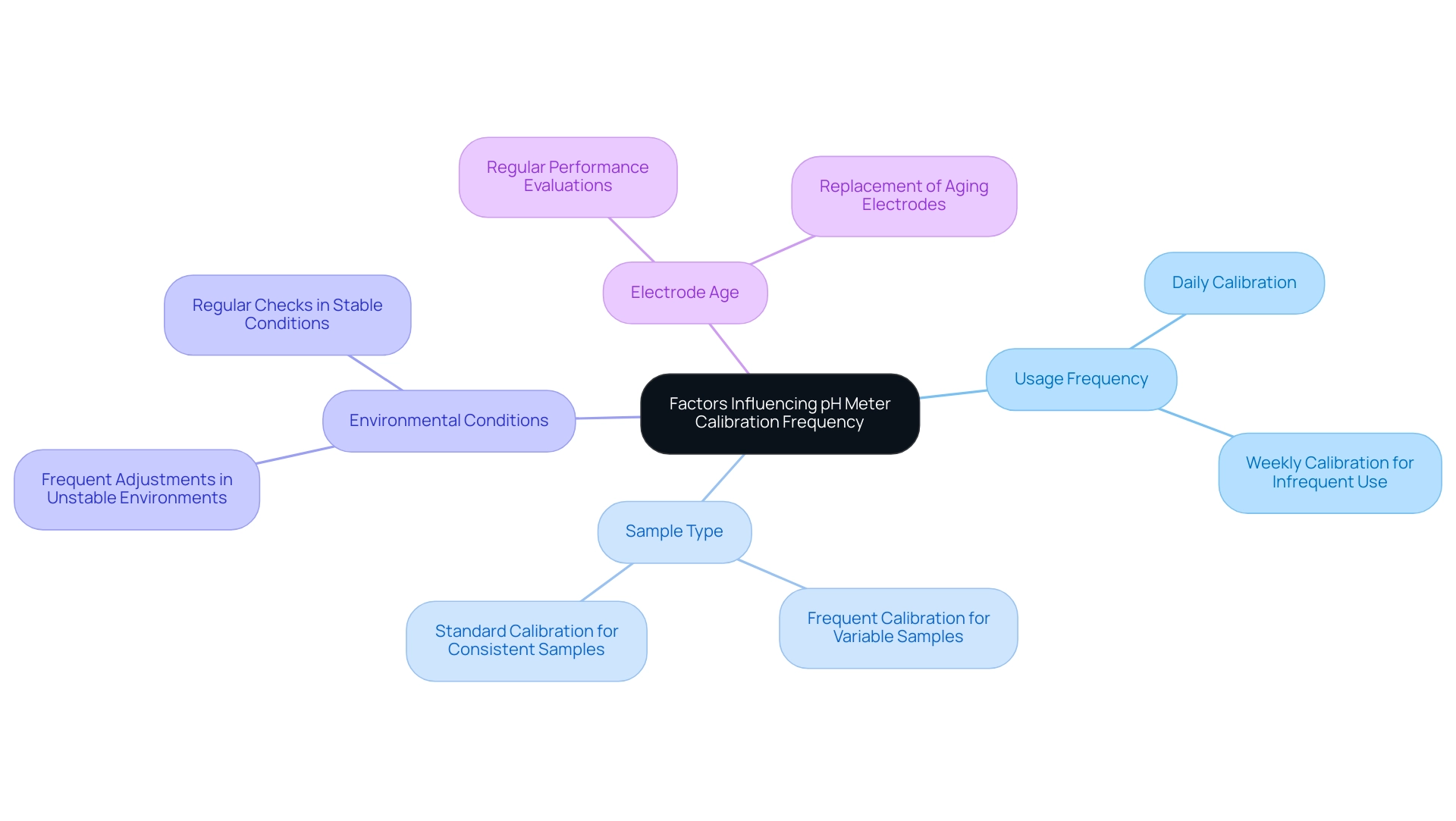
The calibration frequency of pH meters is influenced by several critical factors that demand attention:
- Usage Frequency: For pH meters utilized daily, calibrating before each use is essential to uphold accuracy. Conversely, devices used infrequently should be calibrated at least once a week to ensure reliable measurements.
- Sample Type: When dealing with samples that exhibit significant variability, more frequent calibrations are necessary. This practice mitigates discrepancies arising from fluctuating sample properties, thereby ensuring consistent accuracy in readings.
- Environmental Conditions: The calibration frequency must also account for the environmental conditions in which the pH meter operates. Variations in temperature and humidity can significantly impact pH readings, necessitating more frequent adjustments in unstable environments to maintain measurement integrity.
- Electrode Age: The age of the electrode is another crucial factor in determining adjustment frequency. Older electrodes may experience drift in accuracy, requiring more frequent assessment checks. Regular evaluation of electrode performance is vital, with replacement occurring as needed to sustain accuracy.
In practice, many laboratories establish calibration schedules based on their specific needs. For instance, a pharmaceutical laboratory may perform daily adjustments due to the critical nature of its measurements, while a research facility might opt for weekly adjustments, tailoring the frequency to the particular samples being analyzed.
Moreover, maintaining an adequate supply of buffer solutions is imperative for effective pH probe adjustment, as understanding how to calibrate a pH meter is fundamental for obtaining precise results. A reliable method for verifying whether a pH device requires re-calibration involves using standard reference solutions to measure pH readings, as highlighted in the case study titled 'Checking pH Device Status.' This straightforward check enables users to ascertain how to calibrate a pH meter, ensuring it remains in optimal condition for accurate readings.
Expert opinions suggest that implementing a proactive adjustment schedule not only enhances accuracy and reliability but also extends the lifespan of the equipment. It is advisable to to ensure optimal measurement performance. Furthermore, it is crucial to recognize that the common pH electrode glass in water does not register zero at pH 7.0; rather, it is more negative, which can affect calibration accuracy.
Ultimately, these practices lead to more accurate and trustworthy results.
Conclusion
The precision of pH measurements is critical across various scientific and industrial fields, making the understanding of pH meters essential for achieving reliable results. This article has explored the fundamental components of pH meters, the importance of regular calibration, and the best practices for maintaining these instruments. From the basic functionality of pH meters to the detailed calibration techniques, each aspect plays a vital role in ensuring measurement accuracy.
Calibration, whether through single-point or multi-point methods, is imperative for aligning pH readings with established standards. Regular calibration not only enhances the reliability of results but also prolongs the lifespan of the pH meter. Selecting the appropriate calibration solutions and buffers is equally crucial, as the integrity of these materials directly impacts measurement precision. Additionally, troubleshooting common calibration issues and adhering to maintenance best practices can mitigate potential problems, ensuring optimal performance in laboratory settings.
In conclusion, the reliability of pH measurements hinges on a comprehensive understanding of pH meters, meticulous calibration practices, and consistent maintenance. As technology evolves and the demand for accurate measurements grows, laboratory professionals must prioritize these practices to uphold the integrity of their work. By doing so, they contribute not only to the accuracy of their analyses but also to the advancement of research and quality control in their respective fields.




