Overview
This article centers on mastering the function and maintenance techniques of pH meter electrodes, which are crucial for accurate pH measurement across various scientific applications. Regular cleaning, proper storage, frequent calibration, and careful handling are emphasized as vital practices to ensure that electrodes retain their performance and reliability. Such diligence not only supports the integrity of laboratory analyses but also ensures compliance with industry standards. By adhering to these maintenance techniques, scientists can enhance the accuracy and reliability of their measurements, ultimately contributing to more trustworthy scientific outcomes.
Introduction
Accurate pH measurement stands as a cornerstone of scientific inquiry, influencing a wide array of fields from environmental monitoring to pharmaceutical development. Central to this precision is the pH meter electrode, a sophisticated device that measures acidity or alkalinity while ensuring the reliability of critical data. Despite their importance, many users neglect the essential maintenance techniques required for optimal performance.
Laboratories must consider how to ensure their pH meters deliver consistent and precise results, alongside the best practices that should be employed to extend the life of these indispensable tools.
Explore the Role of pH Meter Electrodes in Measurement
pH sensors are indispensable tools for accurately measuring the acidity or alkalinity of solutions. They consist of a sensing pH meter electrode that detects hydrogen ion concentration and a reference electrode that maintains a stable reference potential. This interaction allows the pH meter to convert voltage differences into precise pH values, which are vital across numerous scientific disciplines, including chemistry, biology, and environmental science. In pharmaceutical laboratories, for example, accurate pH measurements are critical for drug formulation and stability testing, directly influencing product quality and compliance with regulatory standards.
Moreover, the integration of pH readings with Karl Fischer titration techniques, as exemplified by the Hiranuma Aquacounter AQV-300 and AQ-300 titrators, is essential for ensuring the quality and stability of pharmaceutical products. Even minor fluctuations in pH can significantly affect drug efficacy and safety, underscoring the necessity for that respond quickly to temperature changes and maintain high insulation resistance to prevent assessment errors. Recent advancements in pH meter electrode technology, including automated temperature compensation probes, have further enhanced measurement reliability, enabling pharmaceutical manufacturers to optimize formulations and extend shelf life.
By employing effective pH analysis methods in conjunction with Karl Fischer titration, laboratories can protect product quality and meet stringent regulatory requirements, ultimately enhancing patient safety and therapeutic outcomes.
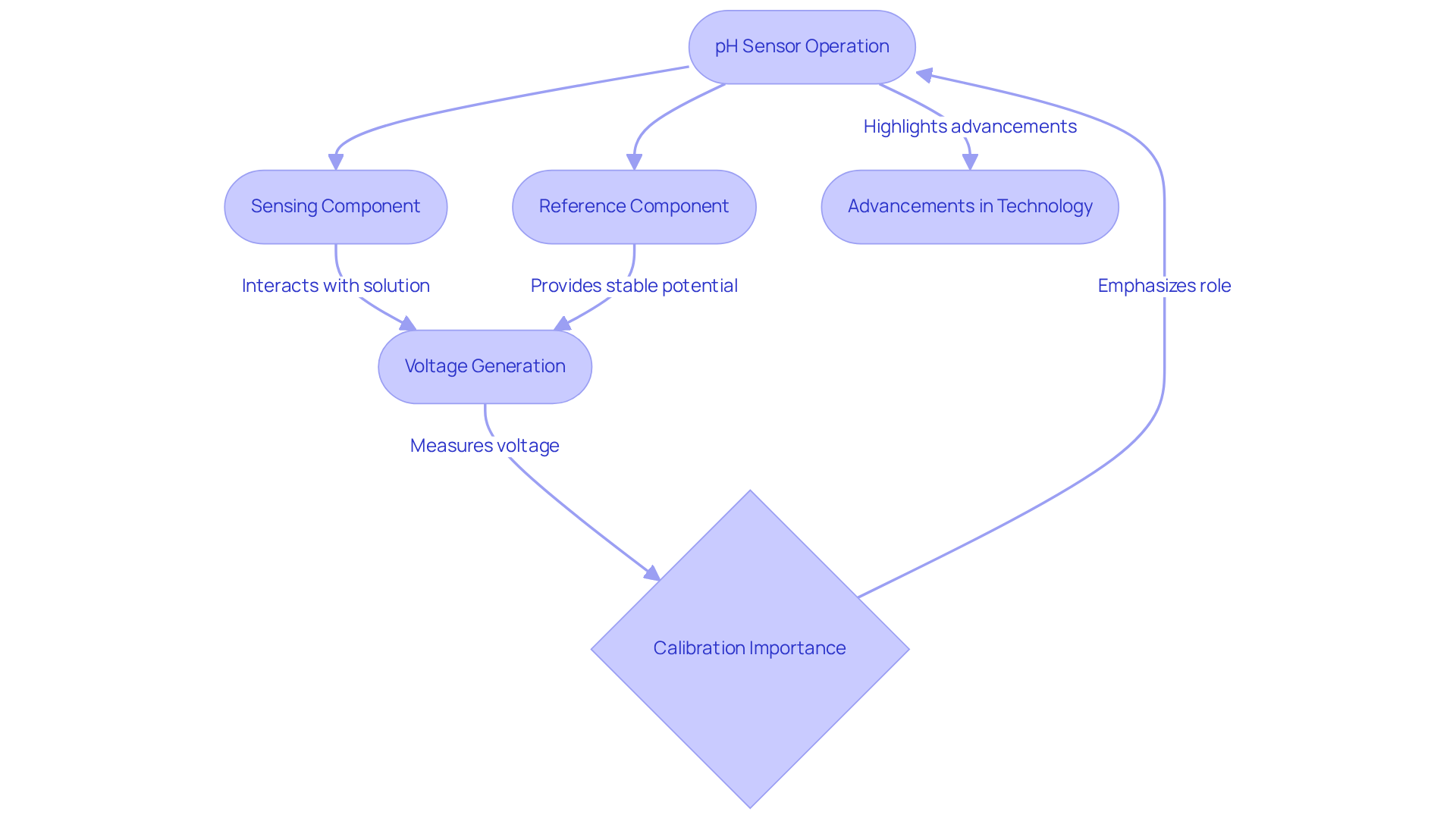
Understand the Mechanism: How pH Electrodes Work
pH sensors operate based on the Nernst equation, establishing a direct relationship between hydrogen ion concentration and the voltage generated by the sensor. The sensing component, typically crafted from glass, interacts with the solution to create a potential difference that accurately reflects hydrogen ion activity. In parallel, the reference component provides a stable potential, enabling the pH device to measure voltage differences with precision. This voltage is subsequently converted into a pH value displayed on the device.
Recent studies highlight the critical importance of calibration in preserving the performance of the pH meter electrode, as even slight deviations can result in substantial measurement errors. can deliver results with a standard uncertainty as low as ±0.043, underscoring the necessity for routine calibration and maintenance. The first commercial pH meter, developed by Arnold Beckman in 1914, marked a pivotal advancement in analytical chemistry, paving the way for modern practices.
Understanding this mechanism not only aids in achieving accurate measurements but also showcases the advancements in pH sensor technology, including enhanced glass formulations and improved reference systems, which contribute to greater accuracy and reliability in laboratory settings, particularly when using a pH meter electrode. The Potentiometric Method remains the most prevalent and dependable design for process control applications when determining pH values. Embracing these technologies is essential for any laboratory aiming to uphold high standards in analytical precision.
Implement Best Practices for pH Electrode Maintenance
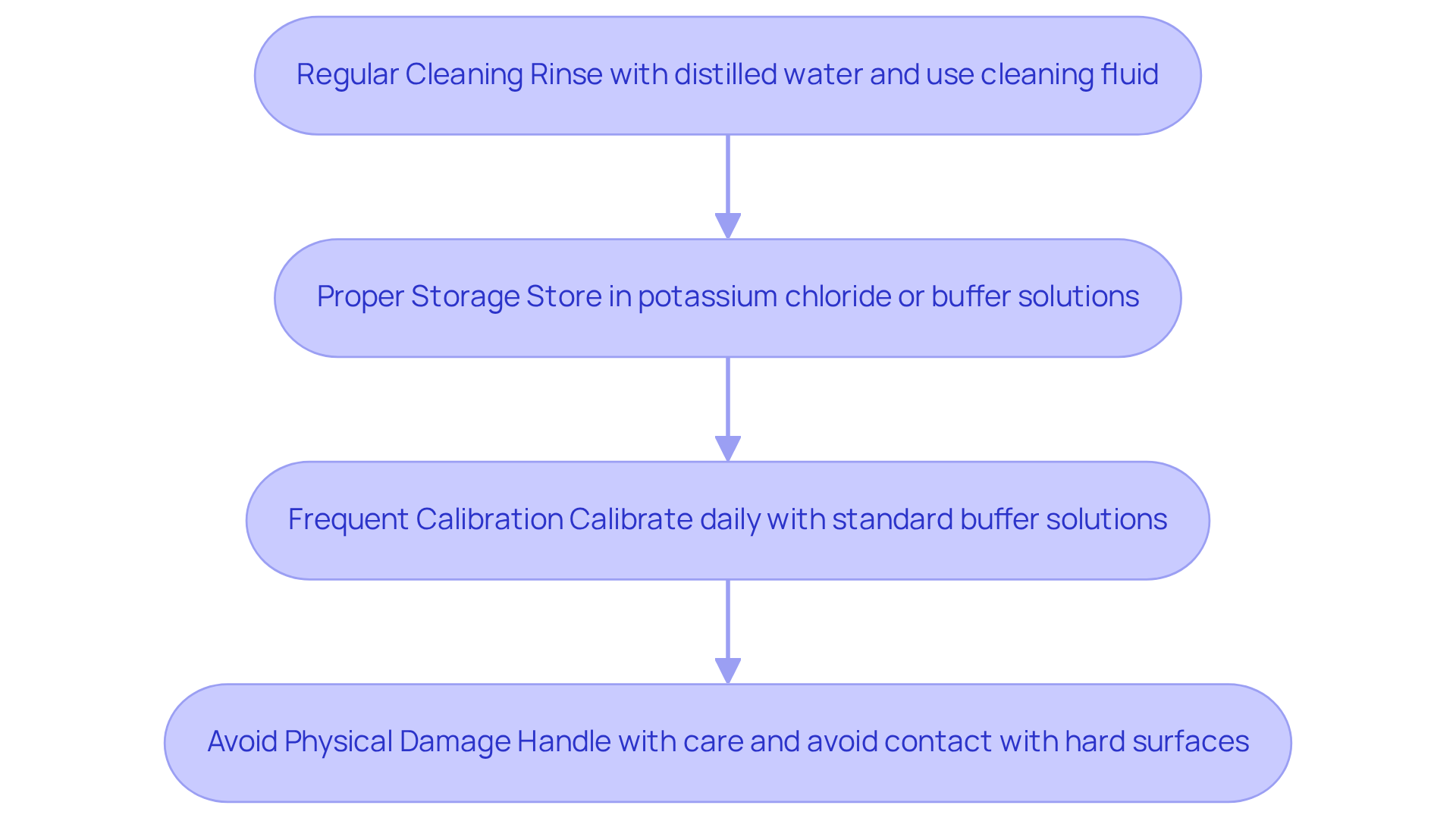
To maintain the pH meter electrode effectively, it is essential to adhere to best practices that ensure accuracy and longevity.
- Regular Cleaning is the first step. After each use, rinse the probe with distilled water to eliminate contaminants. For a comprehensive clean, immerse the sensor in a specialized cleaning fluid for pH sensors for 15-30 minutes, depending on contamination levels. This practice is crucial; improper cleaning can lead to measurement inaccuracies that compromise results.
- Next, consider Proper Storage. Keep the sensor in a manufacturer-recommended storage medium, such as potassium chloride or buffer liquids, rather than distilled water, which can damage the sensor. It is vital to maintain the tip of the conductor moist to prevent drying out, as this can impair functionality.
- Frequent Calibration is another critical aspect. Regular calibration ensures accurate readings. Calibrate daily using at least two standard buffer solutions that encompass the pH range you intend to test, such as pH 4 and pH 7. This calibration should be completed prior to every measurement session, particularly if the device has been inactive for an extended period. Daily calibration is advised to account for potential drift in sensor performance, ensuring reliability in your measurements.
- Finally, Avoid Physical Damage. Handle the component with care to prevent contact with hard surfaces that could crack the glass. Refrain from rubbing the sensor tip with a dry cloth, as this can create static charges that disrupt readings. Instead, ensure the glass bulb and reference junction are completely submerged in the sample during assessments for optimal precision. Using a magnetic stirrer at a low setting is recommended for consistent stirring without damaging the probe.
By following these maintenance practices, users can significantly [extend the lifespan of their pH meter electrode](https://labcompare.com/10-Featured-Articles/593742-LABTips-pH-Probe-Care-and-Maintenance) and ensure consistent measurement accuracy. This ultimately enhances the reliability of laboratory analyses, underscoring the importance of high-quality scientific instruments.
Conclusion
Accurate pH measurement is vital across various scientific disciplines, underscoring the crucial role of pH meter electrodes in delivering reliable data. These sophisticated devices not only measure acidity or alkalinity but also ensure the integrity of scientific research and product quality is maintained. Understanding their function, operation, and maintenance is paramount, as it directly impacts outcomes in fields such as pharmaceuticals and environmental science.
This article delves into the mechanics of pH electrodes, explaining how they operate based on the Nernst equation to provide precise measurements. It highlights the importance of:
- Regular calibration
- Proper cleaning
- Careful handling to enhance the longevity and accuracy of these instruments.
By implementing best practices—such as using appropriate storage solutions and avoiding physical damage—users can ensure that their pH meters remain reliable and effective for their intended applications.
Ultimately, mastery of pH meter electrode function and maintenance is essential for achieving high standards in analytical precision. Laboratories must prioritize these practices to safeguard the quality of their analyses and uphold compliance with rigorous regulatory requirements. Embracing these techniques not only protects product integrity but also enhances overall safety and efficacy in scientific endeavors.




