Overview
This article delves into the critical steps necessary for selecting the appropriate lab data management system (LDMS) specifically designed to meet operational needs in pharmaceutical environments. It underscores the significance of assessing essential features such as:
- Sample tracking
- Data security
- Compliance management
- Integration capabilities
- User-friendly interfaces
These elements are pivotal in enhancing both efficiency and adherence to regulatory standards within laboratory operations. By thoroughly evaluating these aspects, organizations can ensure that their LDMS not only meets current demands but also supports future growth and compliance.
Introduction
Lab data management systems have emerged as indispensable tools for contemporary research facilities, serving as the backbone for efficient data organization and retrieval. These systems not only streamline laboratory workflows but also significantly enhance diagnostic accuracy and compliance within the pharmaceutical and healthcare sectors. However, with a plethora of options available, selecting the appropriate lab data management system can present a formidable challenge. What essential features should laboratories prioritize to ensure they effectively meet both operational needs and regulatory standards?
Understand the Importance of Lab Data Management Systems
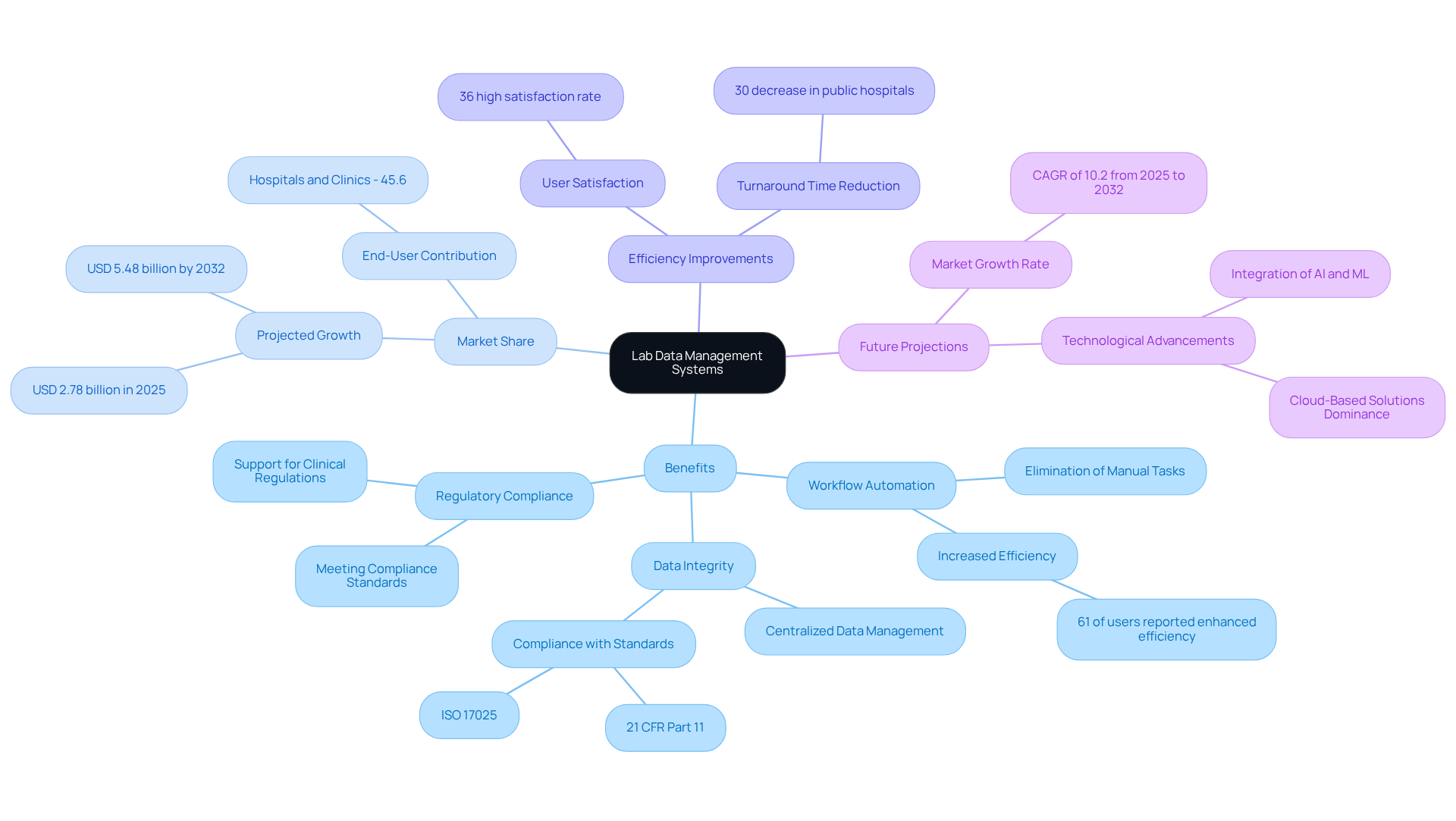
Lab data management systems are indispensable for contemporary research facilities, serving as the backbone for organizing, storing, and retrieving vast quantities of information. These frameworks significantly enhance laboratory efficiency by automating workflows, which minimizes manual errors and fosters improved collaboration among team members. Notably, the integration of the lab data management system with hospital information networks has led to remarkable improvements in diagnostic accuracy and a reduction in turnaround times for medical test reports, achieving over a 30% decrease in public hospitals that adopted these integrated solutions between 2020 and 2022. This enhancement is particularly vital in the pharmaceutical and healthcare sectors, where adherence to regulatory compliance is imperative.
By centralizing data management, the lab data management system (LDMS) not only ensures data integrity but also empowers research facilities to meet rigorous compliance standards while effectively managing extensive datasets. Furthermore, projections indicate that hospitals and clinics will capture the largest market share, accounting for 45.6% of information technology adoption in laboratories by 2025, underscoring the critical role of LDMS in the healthcare industry.
As the demand for precision and reliability in lab operations escalates, comprehending the multifaceted benefits of a lab data management system becomes essential for selecting the optimal setup tailored to the specific needs of your facility. The global information management market is expected to reach USD 5.48 billion by 2032, reflecting the increasing reliance on these platforms. Additionally, 61% of users of information handling platforms have reported enhanced efficiency due to the elimination of manual tasks, further substantiating the claims regarding workflow automation and efficiency improvements.
Identify Key Features for Pharmaceutical Lab Needs
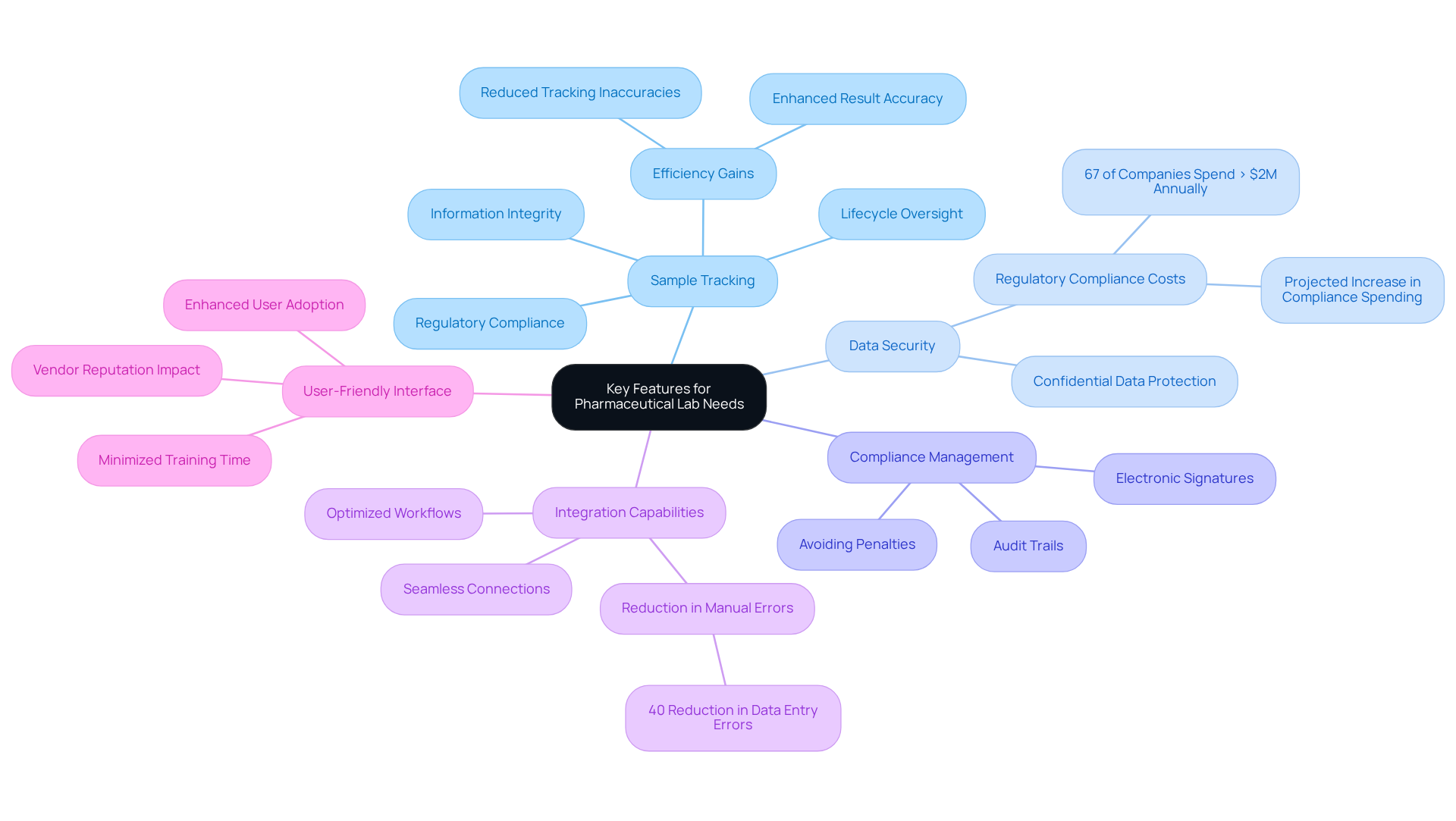
Selecting the right lab data management system for a pharmaceutical facility requires a deliberate assessment of features that cater to specific operational needs. Among the most vital characteristics are:
- Sample Tracking: Effective sample tracking is paramount for overseeing samples throughout their lifecycle—from collection to analysis. This capability is crucial for maintaining information integrity and ensuring compliance with regulatory standards. Laboratories employing advanced LIMS solutions have reported notable enhancements in tracking efficiency, which directly influences result accuracy and overall productivity. Specific examples illustrate that these frameworks can significantly reduce tracking inaccuracies and bolster the reliability of information management processes.
- Data Security: Strong security measures are indispensable. The system must protect confidential data and comply with stringent regulatory standards, particularly in the pharmaceutical sector, where information breaches can have severe consequences. The growing complexity of data privacy regulations has led to 67% of pharmaceutical companies reporting annual compliance expenditures exceeding $2 million, underscoring the critical importance of data security.
- Compliance Management: Features that support adherence to industry regulations, such as audit trails and electronic signatures, are essential. These tools help ensure that facilities meet compliance standards, which is increasingly important as regulatory scrutiny intensifies. Integrating these features is vital for maintaining operational integrity and avoiding costly penalties.
- Integration Capabilities: The ability to seamlessly connect with existing scientific instruments and software is crucial for optimizing workflows. Systems that facilitate integration can greatly reduce manual data entry errors and enhance operational efficiency, as evidenced by case studies demonstrating a 40% reduction in data entry errors in trials utilizing integrated platforms. This integration not only boosts accuracy but also expedites overall laboratory processes.
- User-Friendly Interface: A platform with an intuitive interface enhances user adoption and minimizes training time. Laboratories that emphasize user experience often experience faster implementation and greater overall satisfaction among staff. Furthermore, vendor reputation and brand recognition significantly influence LIMS selection, affecting user confidence and the effectiveness of the solution.
By prioritizing these features, pharmaceutical facilities can select a lab data management system that not only meets their operational requirements but also supports regulatory compliance and enhances overall efficiency.
Compare Available Lab Data Management Systems
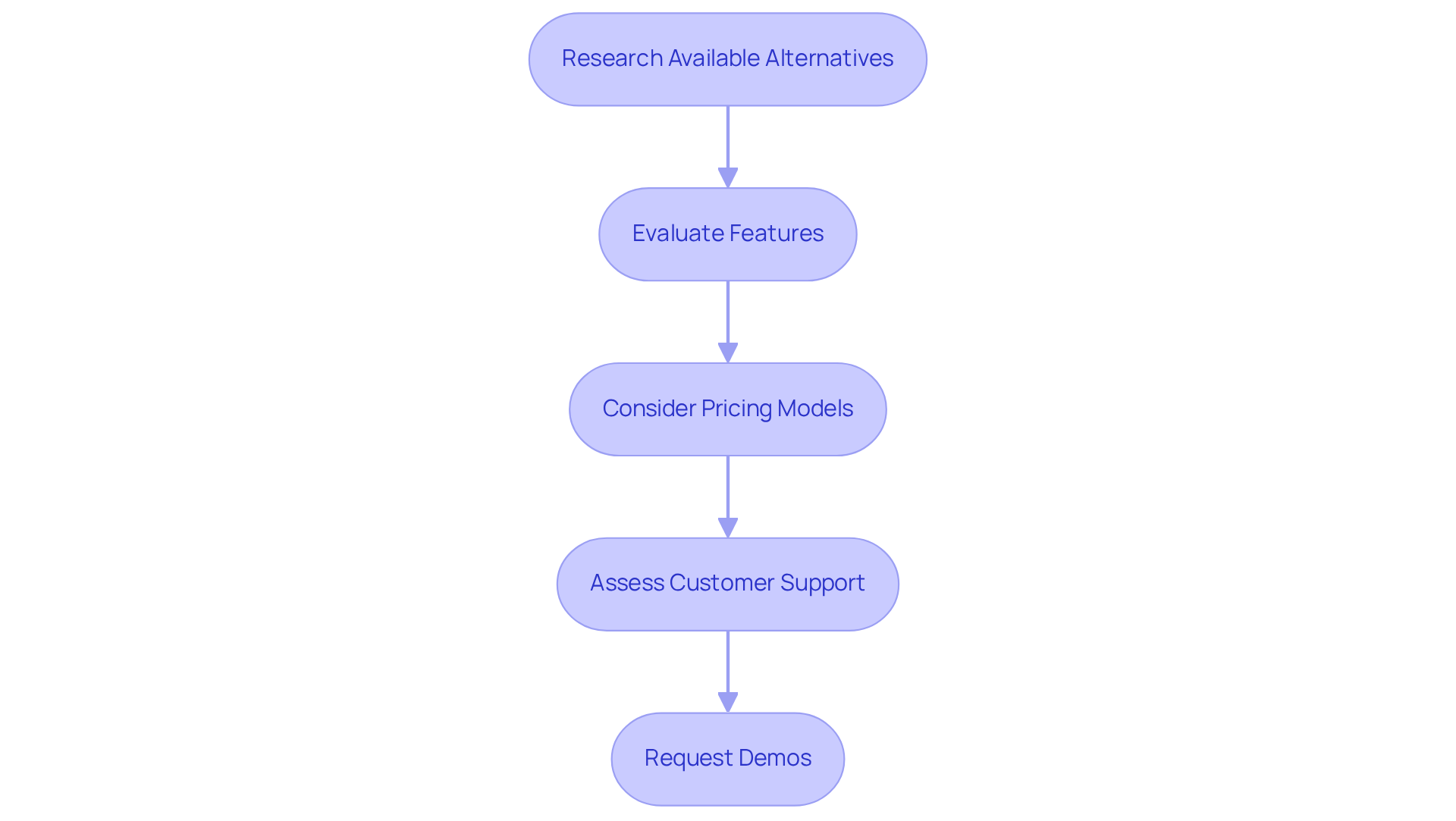
To effectively compare lab data management systems, follow these essential steps:
-
Research Available Alternatives: Begin by compiling a comprehensive list of potential setups tailored to your facility's specific needs. Utilize resources such as industry reviews, user testimonials, and expert recommendations to pinpoint suitable candidates. Notably, over 40% of laboratory decision-makers in emerging markets cite budget constraints as a significant barrier to adopting advanced informatics solutions, underscoring the necessity of thorough research.
-
Evaluate Features: Develop a comparison chart that highlights the critical characteristics of each platform. Pay particular attention to how well each option meets your unique requirements, including functionalities of the lab data management system that enhance data management and compliance. With the LIMS market projected to reach USD 5.26 billion by 2032, grasping the features that drive this growth is imperative.
-
Consider Pricing Models: Scrutinize the pricing structures of each framework, factoring in initial costs, subscription fees, and any additional expenses for upgrades or support. Comprehending these financial elements is vital for effective budgeting, especially considering the high implementation and maintenance costs that can hinder the adoption of a lab data management system in smaller laboratories.
-
Assess Customer Support: Examine the level of customer support offered by each vendor. Dependable support is crucial during both the implementation phase and ongoing usage, ensuring that any issues can be swiftly resolved. Research shows that 62.67% of respondents prioritize application support when selecting a lab data management system, highlighting its importance in the decision-making process.
-
Request Demos: Whenever feasible, request demonstrations or trial versions of the platforms. This hands-on experience enables you to assess usability and functionality, aiding you in making a more informed decision. As the integration of AI, cloud computing, and advanced analytics becomes increasingly prevalent in lab data management systems, evaluating how these technologies are incorporated into the platforms can yield valuable insights.
By adhering to these steps, you can confidently select a laboratory information solution that meets your operational requirements and budget constraints.
Plan for Implementation and Staff Training
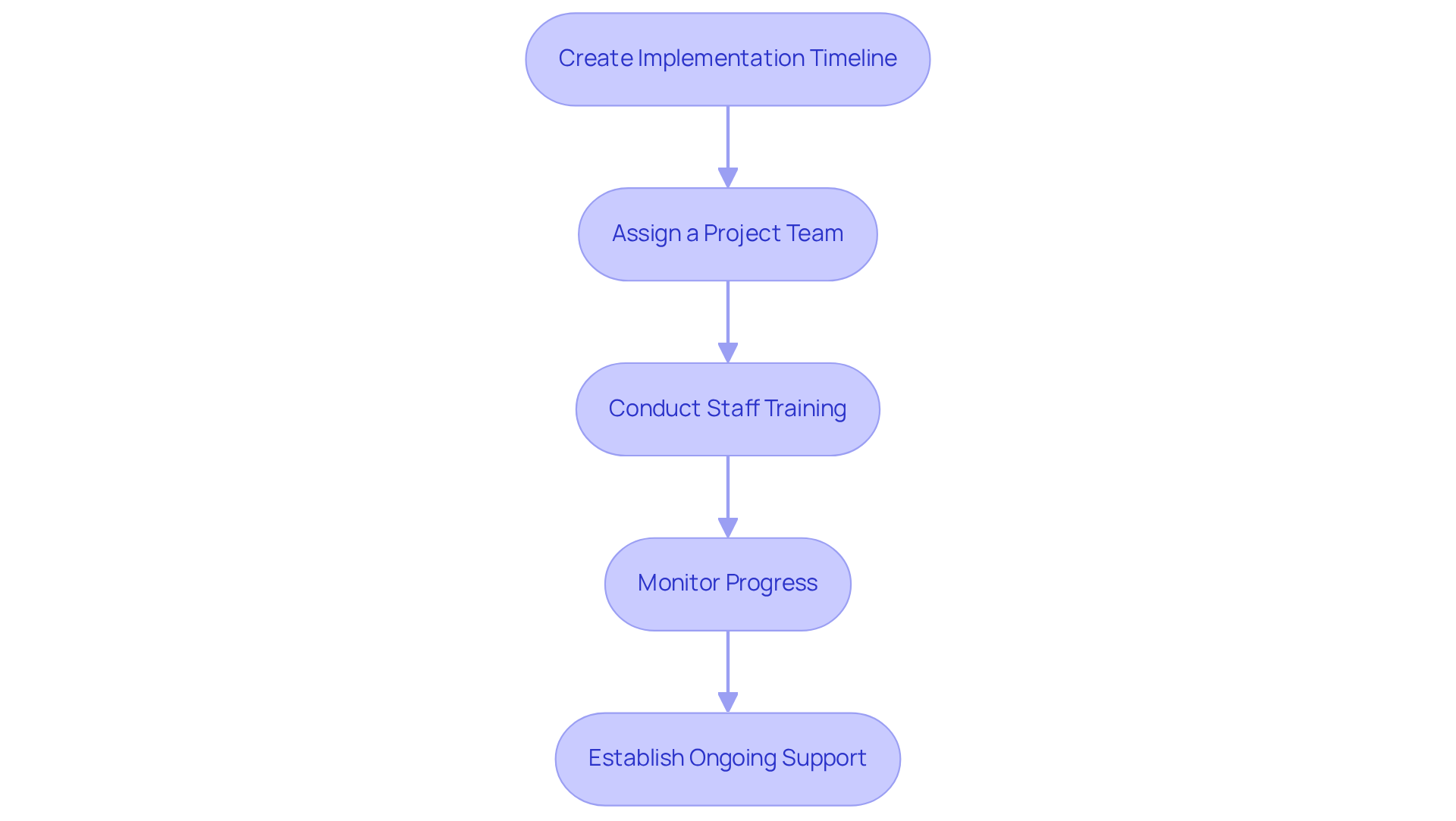
Introducing a new laboratory information management framework necessitates meticulous planning and execution. Essential steps are crucial for ensuring a successful transition:
-
Create an Implementation Timeline: Establish a detailed schedule that outlines each phase of the implementation process, including information transfer, configuration setup, and thorough testing. This structured approach aids in managing expectations and ensuring timely completion.
-
Assign a Project Team: Form a dedicated team responsible for overseeing the implementation. This team should encompass representatives from various departments, ensuring that diverse perspectives and needs are addressed throughout the process.
-
Conduct Staff Training: Develop a robust training program tailored to different user roles. Effective training should encompass operational functionalities, data entry procedures, and compliance requirements. Research indicates that well-structured training enhances staff skills and performance, leading to improved operational efficiency and compliance with quality standards. Notably, LIMS training programs offer flexibility, allowing staff to access materials at their own pace, which is essential for accommodating busy schedules.
-
Monitor Progress: Continuously evaluate the implementation process and proactively address any challenges that arise. Consistent input from users is vital for recognizing areas requiring enhancement, ensuring that the setup fulfills the laboratory's operational requirements. Defining roles and performance metrics during this phase can assist in monitoring overall performance and identifying further training needs.
-
Establish Ongoing Support: Provide users with access to continuous support and resources, such as user manuals and helpdesk services. This ongoing support fosters continuous learning and problem-solving, empowering staff to utilize the platform effectively. Organizations such as Broward Health have demonstrated the advantages of continuous support, realizing substantial cost reductions through efficient lab oversight practices.
By adhering to these steps, laboratories can successfully implement their chosen lab data management system, thereby enhancing operational efficiency and empowering staff to leverage the full potential of the system.
Conclusion
The selection of an effective lab data management system is essential for enhancing the operational efficiency of laboratories, particularly within the pharmaceutical sector. Recognizing the significance of these systems allows facilities to streamline their data management processes, improve compliance with regulatory standards, and ultimately foster better collaboration and accuracy in research and diagnostics.
Key features such as:
- Sample tracking
- Data security
- Compliance management
- Integration capabilities
- User-friendly interfaces
are critical when choosing the right lab data management system. A systematic approach to comparing available systems and planning for implementation empowers laboratories to make informed decisions tailored to their specific needs. Moreover, emphasizing staff training and ongoing support highlights the necessity of equipping users with the skills needed to maximize the system’s potential.
Investing time and resources into selecting and implementing a lab data management system transcends a mere technical upgrade; it represents a strategic initiative that can significantly enhance laboratory operations. As the landscape of healthcare evolves, embracing these systems will be vital for maintaining competitiveness and ensuring compliance with ever-increasing regulatory demands. Laboratories are strongly encouraged to prioritize these systems to unlock their full potential and drive innovation in research and patient care.




